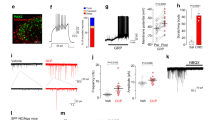
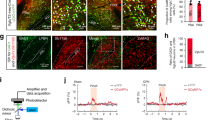
Abstract
Dynorphin A is an endogenous opioid peptide that produces non-opioid receptor-mediated neural excitation. Here we demonstrate that dynorphin induces calcium influx via voltage-sensitive calcium channels in sensory neurons by activating bradykinin receptors. This action of dynorphin at bradykinin receptors is distinct from the primary signaling pathway activated by bradykinin and underlies the hyperalgesia produced by pharmacological administration of dynorphin by the spinal route in rats and mice. Blockade of spinal B1 or B2 receptor also reverses persistent neuropathic pain but only when there is sustained elevation of endogenous spinal dynorphin, which is required for maintenance of neuropathic pain. These data reveal a mechanism for endogenous dynorphin to promote pain through its agonist action at bradykinin receptors and suggest new avenues for therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Beaumont, A. & Hughes, J. Biology of opioid peptides. Annu. Rev. Pharmacol. Toxicol. 19, 245–267 (1979).
Goldstein, A., Tachibana, S., Lowney, L.I., Hunkapiller, M. & Hood, L. Dynorphin-(1–13), an extraordinarily potent opioid peptide. Proc. Natl. Acad. Sci. USA 76, 6666–6670 (1979).
Quirion, R. & Pilapil, C. Opioid receptor binding profile of [3H]dynorphin A-(1–8) in rat and guinea pig brain. Eur. J. Pharmacol. 99, 361–363 (1984).
Chavkin, C., James, I.F. & Goldstein, A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215, 413–415 (1982).
Corbett, A.D., Paterson, S.J., McKnight, A.T., Magnan, J. & Kosterlitz, H.W. Dynorphin and dynorphin are ligands for the kappa-subtype of opiate receptor. Nature 299, 79–81 (1982).
Zhang, S. et al. Dynorphin A as a potential endogenous ligand for four members of the opioid receptor gene family. J. Pharmacol. Exp. Ther. 286, 136–141 (1998).
Herman, B.H. & Goldstein, A. Antinociception and paralysis induced by intrathecal dynorphin A. J. Pharmacol. Exp. Ther. 232, 27–32 (1985).
Walker, J.M., Moises, H.C., Coy, D.H., Baldrighi, G. & Akil, H. Nonopiate effects of dynorphin and des-Tyr-dynorphin. Science 218, 1136–1138 (1982).
Faden, A.I. & Jacobs, T.P. Dynorphin-related peptides cause motor dysfunction in the rat through a non-opiate action. Br. J. Pharmacol. 81, 271–276 (1984).
Stevens, C.W., Weinger, M.B. & Yaksh, T.L. Intrathecal dynorphins suppress hindlimb electromyographic activity in rats. Eur. J. Pharmacol. 138, 299–302 (1987).
Vanderah, T.W. et al. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain 68, 275–281 (1996).
Koetzner, L., Hua, X.Y., Lai, J., Porreca, F. & Yaksh, T. Nonopioid actions of intrathecal dynorphin evoke spinal excitatory amino acid and prostaglandin E2 release mediated by cyclooxygenase-1 and -2. J. Neurosci. 24, 1451–1458 (2004).
Skilling, S.R., Sun, X., Kurtz, H.J. & Larson, A.A. Selective potentiation of NMDA-induced activity and release of excitatory amino acids by dynorphin: possible roles in paralysis and neurotoxicity. Brain Res. 575, 272–278 (1992).
Tang, Q., Lynch, R.M., Porreca, F. & Lai, J. Dynorphin A elicits an increase in intracellular calcium in cultured neurons via a non-opioid, non-NMDA mechanism. J. Neurophysiol. 83, 2610–2615 (2000).
Hauser, K.F., Foldes, J.K. & Turbek, C.S. Dynorphin A (1–13) neurotoxicity in vitro: opioid and non-opioid mechanisms in mouse spinal cord neurons. Exp. Neurol. 160, 361–375 (1999).
Ruda, M.A., Iadarola, M.J., Cohen, L.V. & Young, W.S., III . In situ hybridization histochemistry and immunocytochemistry reveal an increase in spinal dynorphin biosynthesis in a rat model of peripheral inflammation and hyperalgesia. Proc. Natl. Acad. Sci. USA 85, 622–626 (1988).
Malan, T.P. et al. Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain 86, 185–194 (2000).
Schwei, M.J. et al. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J. Neurosci. 19, 10886–10897 (1999).
Vanderah, T.W. et al. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J. Neurosci. 20, 7074–7079 (2000).
Wang, Z. et al. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J. Neurosci. 21, 1779–1786 (2001).
Platika, D., Boulos, M.H., Baizer, L. & Fishman, M.C. Neuronal traits of clonal cell lines derived by fusion of dorsal root ganglia neurons with neuroblastoma cells. Proc. Natl Acad. Sci. USA 82, 3499–3503 (1985).
Francel, P.C. et al. Neurochemical characteristics of a novel dorsal root ganglion X neuroblastoma hybrid cell line, F-11. J. Neurochem. 48, 1624–1631 (1987).
Fan, S.F., Shen, K.F., Scheideler, M.A. & Crain, S.M. F11 neuroblastoma x DRG neuron hybrid cells express inhibitory mu- and delta-opioid receptors which increase voltage-dependent K+ currents upon activation. Brain Res. 590, 329–333 (1992).
Zanner, R., Hapfelmeier, G., Gratzl, M. & Prinz, C. Intracellular signal transduction during gastrin-induced histamine secretion in rat gastric ECL cells. Am. J. Physiol. Cell Physiol. 282, C374–C382 (2002).
Simasko, S.M., Boyadjieva, N., De, A. & Sarkar, D.K. Effect of ethanol on calcium regulation in rat fetal hypothalamic cells in culture. Brain Res. 824, 89–96 (1999).
Burgess, G.M., Mullaney, I., McNeill, M., Dunn, P.M. & Rang, H.P. Second messengers involved in the mechanism of action of bradykinin in sensory neurons in culture. J. Neurosci. 9, 3314–3325 (1989).
Tsien, R.W., Lipscombe, D., Madison, D.V., Bley, K.R. & Fox, A.P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 11, 431–438 (1988).
Davare, M.A., Dong, F., Rubin, C.S. & Hell, J.W. The A-kinase anchor protein MAP2B and cAMP-dependent protein kinase are associated with class C L-type calcium channels in neurons. J. Biol. Chem. 274, 30280–30287 (1999).
Hell, J.W., Yokoyama, C.T., Breeze, L.J., Chavkin, C. & Catterall, W.A. Phosphorylation of presynaptic and postsynaptic calcium channels by cAMP-dependent protein kinase in hippocampal neurons. EMBO J. 14, 3036–3044 (1995).
Wetzel, C.H., Spehr, M. & Hatt, H. Phosphorylation of voltage-gated ion channels in rat olfactory receptor neurons. Eur. J. Neurosci. 14, 1056–1064 (2001).
Liebmann, C. et al. Dual bradykinin B2 receptor signalling in A431 human epidermoid carcinoma cells: activation of protein kinase C is counteracted by a GS-mediated stimulation of the cyclic AMP pathway. Biochem. J. 313, 109–118 (1996).
Marceau, F., Hess, J.F. & Bachvarov, D.R. The B1 receptors for kinins. Pharmacol. Rev. 50, 357–386 (1998).
Austin, C.E. et al. Stable expression of the human kinin B1 receptor in Chinese hamster ovary cells. Characterization of ligand binding and effector pathways. J. Biol. Chem. 272, 11420–11425 (1997).
Vasko, M.R., Campbell, W.B. & Waite, K.J. Prostaglandin E2 enhances bradykinin-stimulated release of neuropeptides from rat sensory neurons in culture. J. Neurosci. 14, 4987–4997 (1994).
Evans, A.R., Nicol, G.D. & Vasko, M.R. Differential regulation of evoked peptide release by voltage-sensitive calcium channels in rat sensory neurons. Brain Res. 712, 265–273 (1996).
Linhart, O., Obreja, O. & Kress, M. The inflammatory mediators serotonin, prostaglandin E2 and bradykinin evoke calcium influx in rat sensory neurons. Neuroscience 118, 69–74 (2003).
Falcone, R.C. et al. Characterization of bradykinin receptors in guinea pig gall bladder. J. Pharmacol. Exp. Ther. 266, 1291–1299 (1993).
Pesquero, J.B. et al. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc. Natl Acad. Sci. USA 97, 8140–8145 (2000).
Banik, R.K., Kozaki, Y., Sato, J., Gera, L. & Mizumura, K. B2 receptor-mediated enhanced bradykinin sensitivity of rat cutaneous C-fiber nociceptors during persistent inflammation. J. Neurophysiol. 86, 2727–2735 (2001).
Wotherspoon, G. & Winter, J. Bradykinin B1 receptor is constitutively expressed in the rat sensory nervous system. Neurosci. Lett. 294, 175–178 (2000).
Fox, A. et al. Regulation and function of spinal and peripheral neuronal B1 bradykinin receptors in inflammatory mechanical hyperalgesia. Pain 104, 683–691 (2003).
Burgess, S.E. et al. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J. Neurosci. 22, 5129–5136 (2002).
Kim, S.H. & Chung, J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50, 355–363 (1992).
Gardell, L.R. et al. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J. Neurosci. 22, 6747–6755 (2002).
Cloutier, F., de Sousa Buck, H., Ongali, B. & Couture, R. Pharmacologic and autoradiographic evidence for an up-regulation of kinin B(2) receptors in the spinal cord of spontaneously hypertensive rats. Br. J. Pharmacol. 135, 1641–1654 (2002).
Botticelli, L.J., Cox, B.M. & Goldstein, A. Immunoreactive dynorphin in mammalian spinal cord and dorsal root ganglia. Proc. Natl Acad. Sci. USA 78, 7783–7786 (1981).
Lai, J. et al. The cloned murine M1 muscarinic receptor is associated with the hydrolysis of phosphatidylinositols in transfected murine B82 cells. Life Sci. 42, 2489–2502 (1988).
Yaksh, T.L. & Rudy, T.A. Chronic catheterization of the spinal subarachnoid space. Physiol. Behav. 17, 1031–1036 (1976).
Chaplan, S.R., Bach, F.W., Pogrel, J.W., Chung, J.M. & Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994).
Hargreaves, K., Dubner, R., Brown, F., Flores, C. & Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88 (1988).
Acknowledgements
This work was supported by a grant from the National Institute on Drug Abuse. The authors appreciate the technical assistance of Y. Kawamoto on DRG cultures and H. Badghisi on the PI hydrolysis assay.
Author information
Authors and Affiliations
Contributions
M.-C.L. conducted the ratiometric imaging, designed and executed the PCR analyses, performed the data analysis and prepared the figures. Q.C. conducted the surgeries and behavioral experiments and data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
F-11 cells exhibit characteristics of nociceptive C-fibers. (PDF 2126 kb)
Supplementary Fig. 2
Voltage-sensitive calcium channels (VSCCs) are expressed in F-11 cells. (PDF 5352 kb)
Supplementary Fig. 3
Dynorphin A(2-13) has very low affinity for the cloned opioid receptors. (PDF 2764 kb)
Supplementary Fig. 4
Heterologous expression of the human bradykinin B1 or B2 receptor in F-11 cells. (PDF 4479 kb)
Supplementary Fig. 5
HOE 140 blocks dynorphin A(2-13)-induced [Ca2+]i in transfected F-11 expressing the human B2 receptor. (PDF 2362 kb)
Rights and permissions
About this article
Cite this article
Lai, J., Luo, MC., Chen, Q. et al. Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nat Neurosci 9, 1534–1540 (2006). https://doi.org/10.1038/nn1804
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1804
This article is cited by
-
Detection of dynorphin 1-17 biotransformation fragments in human nasal polyps by UPLC-QTOF-MS
Analytical and Bioanalytical Chemistry (2024)
-
Alkoholkonsum und chronische Schmerzen - ein Update
Schmerzmedizin (2023)
-
Bradykinin Receptors Play a Critical Role in the Chronic Post-ischaemia Pain Model
Cellular and Molecular Neurobiology (2021)
-
The atypical chemokine receptor ACKR3/CXCR7 is a broad-spectrum scavenger for opioid peptides
Nature Communications (2020)