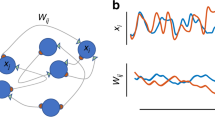
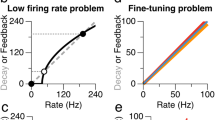
Abstract
Hippocampal area CA3 is widely considered to function as an autoassociative memory. However, it is insufficiently understood how it does so. In particular, the extensive experimental evidence for the importance of carefully regulated spiking times poses the question as to how spike timing–based dynamics may support memory functions. Here, we develop a normative theory of autoassociative memory encompassing such network dynamics. Our theory specifies the way that the synaptic plasticity rule of a memory constrains the form of neuronal interactions that will retrieve memories optimally. If memories are stored by spike timing–dependent plasticity, neuronal interactions should be formalized in terms of a phase response curve, indicating the effect of presynaptic spikes on the timing of postsynaptic spikes. We show through simulation that such memories are competent analog autoassociators and demonstrate directly that the attributes of phase response curves of CA3 pyramidal cells recorded in vitro qualitatively conform with the theory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Squire, L.R. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99, 195–231 (1992).
Cohen, N.J. & Eichenbaum, H. Memory, Amnesia, and the Hippocampal System (MIT Press, Cambridge, Massachusetts, 1993).
Rolls, E.T. & Treves, A. Neural Networks and Brain Function (Oxford Univ. Press, Oxford, 1998).
Amaral, D.G., Ishizuka, N. & Claiborne, B. Neurons, numbers and the hippocampal network. Prog. Brain Res. 83, 1–11 (1990).
Hopfield, J.J. Neural networks and physical systems with emergent collective computational abilities. Proc. Natl. Acad. Sci. USA 79, 2554–2558 (1982).
Baird, B. Bifurcation and learning in network models of oscillating cortex. Physica D. 42, 365–384 (1990).
Wang, D.L., Buhmann, J. & von der Malsburg, C. Pattern segmentation in associative memory. Neural Comput. 2, 94–106 (1990).
Li, Z. Modeling the sensory computations of the olfactory bulb. in Models of Neural Networks Vol. 2 (eds. Domany, E., van Hemmen, J.L. & Schulten, K.) 221–251 (Springer-Verlag, New York, 1995).
Hendin, O., Horn, D. & Tsodyks, M.V. Associative memory and segmentation in an oscillatory neural model of the olfactory bulb. J. Comput. Neurosci. 5, 157–169 (1998).
Li, Z. & Hertz, J. Odour recognition and segmentation by a model olfactory bulb and cortex. Network 11, 83–102 (2000).
Hasselmo, M.E., Bodelon, C. & Wyble, B.P. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 14, 793–817 (2002).
Scarpetta, S., Li, Z. & Hertz, J. Hebbian imprinting and retrieval in oscillatory neural networks. Neural Comput. 14, 2371–2396 (2002).
Jensen, O. & Lisman, J. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 28, 67–72 (2005).
Hopfield, J.J. Neurons with graded response have collective computational properties like those of two-state neurons. Proc. Natl. Acad. Sci. USA 81, 3088–3092 (1984).
Treves, A. Graded-response neurons and information encodings in autoassociative memories. Phys. Rev. A. 42, 2418–2430 (1990).
O'Keefe, J. & Recce, M.L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330 (1993).
Poe, G.R., Nitz, D.A., McNaughton, B.L. & Barnes, C.A. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 855, 176–180 (2000).
Skaggs, W.E., McNaughton, B.L., Wilson, M.A. & Barnes, C.A. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172 (1996).
Harris, K.D., Csicsvári, J., Hirase, H., Dragoi, G. & Buzsáki, G. Organization of cell assemblies in the hippocampus. Nature 424, 552–556 (2003).
Skaggs, W.E. & McNaughton, B.L. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271, 1870–1873 (1996).
Nádasdy, Z., Hirase, H., Czurkó, A., Csicsvári, J. & Buzsáki, G. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19, 9497–9507 (1999).
Louie, K. & Wilson, M.A. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron 29, 145–156 (2001).
Paulsen, O. & Sejnowski, T.J. Natural patterns of activity and long-term synaptic plasticity. Curr. Opin. Neurobiol. 10, 172–179 (2000).
Bi, G. & Poo, M.M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu. Rev. Neurosci. 24, 139–166 (2001).
Rinzel, J. & Ermentrout, B. Analysis of neural excitability and oscillations. in Methods in Neuronal Modeling 2nd edn. (eds. Koch, C. & Segev, I.) 251–291 (MIT Press, Cambridge, Massachusetts, 1998).
Brown, E., Moehlis, J. & Holmes, P. On the phase reduction and response dynamics of neural oscillator populations. Neural Comput. 16, 673–715 (2004).
Gutkin, B.S., Ermentrout, G.B. & Reyes, A. Phase response curves determine the responses of neurons to transient inputs. J. Neurophysiol. 94, 1623–1635 (2005).
Guevara, M.R., Glass, L. & Shrier, A. Phase locking, period-doubling bifurcations, and irregular dynamics in periodically stimulated cardiac cells. Science 214, 1350–1353 (1981).
Ermentrout, B. & Kopell, N. Learning of phase lags in coupled neural oscillators. Neural Comput. 6, 225–241 (1994).
Lampl, I. & Yarom, Y. Subthreshold oscillations of the membrane potential: a functional synchronizing and timing device. J. Neurophysiol. 70, 2181–2186 (1993).
Ermentrout, G.B. & Kopell, N. Fine structure of neural spiking and synchronization in the presence of conduction delays. Proc. Natl. Acad. Sci. USA 95, 1259–1264 (1998).
MacKay, D.J.C. Maximum entropy connections: neural networks. in Maximum Entropy and Bayesian Methods, Laramie, 1990 (eds. Grandy, Jr, W.T. & Schick, L.H.) 237–244 (Kluwer, Dordrecht, The Netherlands, 1991).
Sommer, F.T. & Dayan, P. Bayesian retrieval in associative memories with storage errors. IEEE Trans. Neural Netw. 9, 705–713 (1998).
Bi, G.Q. & Poo, M.M. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci. 18, 10464–10472 (1998).
Buzsáki, G. Theta oscillations in the hippocampus. Neuron 33, 325–340 (2002).
Buzsáki, G. & Draguhn, A. Neuronal oscillations in cortical networks. Science 304, 1926–1929 (2004).
Abbott, L.F. & Nelson, S.B. Synaptic plasticity: taming the beast. Nat. Neurosci. 3, 1178–1183 (2000).
Ermentrout, B. Type I membranes, phase resetting curves, and synchrony. Neural Comput. 8, 979–1001 (1996).
Engel, A., Englisch, H. & Schütte, A. Improved retrieval in neural networks with external fields. Europhys. Lett. 8, 393–399 (1989).
Reyes, A.D. & Fetz, F.E. Effects of transient depolarizing potentials on the firing rate of cat neocortical neurons. J. Neurophysiol. 69, 1673–1683 (1993).
Hasselmo, M.E. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn. Sci. 3, 351–359 (1999).
Froemke, R.C. & Dan, Y. Spike-timing-dependent synaptic modification induced by natural spike trains. Nature 416, 433–438 (2002).
Sjostrom, P.J., Turrigiano, G.G. & Nelson, S.B. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron 32, 1149–1164 (2001).
Lengyel, M., Szatmáry, Z. & Érdi, P. Dynamically detuned oscillations account for the coupled rate and temporal code of place cell firing. Hippocampus 13, 700–714 (2003).
Huxter, J., Burgess, N. & O'Keefe, J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature 425, 828–832 (2003).
Huhn, Z., Orbán, G., Érdi, P. & Lengyel, M. Theta oscillation-coupled dendritic spiking integrates inputs on a long time scale. Hippocampus 15, 950–962 (2005).
Siapas, A.G. & Wilson, M.A. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21, 1123–1128 (1998).
Sirota, A., Csicsvári, J., Buhl, D. & Buzsáki, G. Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl. Acad. Sci. USA 100, 2065–2069 (2003).
Csicsvári, J., Hirase, H., Czurkó, A., Mamiya, A. & Buzsáki, G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J. Neurosci. 19, 274–287 (1999).
Siapas, A.G., Lubenov, E.V. & Wilson, M.A. Prefrontal phase locking to hippocampal theta oscillations. Neuron 46, 141–151 (2005).
Acknowledgements
We thank B. Gutkin, D. MacKay and E. Shea-Brown for valuable discussions and E.O. Mann and D. McLelland for their help with Igor procedures. This work was supported by the Gatsby Charitable Foundation (M.L., P.D.), the European Bayesian-Inspired Brain and Artefacts project (M.L., P.D.), the Biotechnology and Biological Sciences Research Council (J.K., O.P.), the Kwanjung Educational Foundation, Korea (J.K.) and the Oxford University Clarendon Fund (J.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Recall performance in adversarial settings. (PDF 185 kb)
Supplementary Fig. 2
Consequences of a broad, smoothly varying STDP curve on the optimal coupling function and phase response curves. (PDF 165 kb)
Supplementary Fig. 3
Effect of increased oscillatory frequency on the PRC. (PDF 359 kb)
Supplementary Fig. 4
Burst-based PRCs. (PDF 448 kb)
Rights and permissions
About this article
Cite this article
Lengyel, M., Kwag, J., Paulsen, O. et al. Matching storage and recall: hippocampal spike timing–dependent plasticity and phase response curves. Nat Neurosci 8, 1677–1683 (2005). https://doi.org/10.1038/nn1561
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1561
This article is cited by
-
Advances in the computational understanding of mental illness
Neuropsychopharmacology (2021)
-
Design principles for enhancing phase sensitivity and suppressing phase fluctuations simultaneously in biochemical oscillatory systems
Nature Communications (2018)
-
Origins and suppression of oscillations in a computational model of Parkinson’s disease
Journal of Computational Neuroscience (2014)
-
Optimal pair of hippocampal CA1 phase response curve and spike-timing-dependent plasticity for hetero-associative memory
BMC Neuroscience (2013)
-
Network topology and intrinsic excitability of the existing network drive integration patterns in a model of adult neurogenesis
BMC Neuroscience (2013)