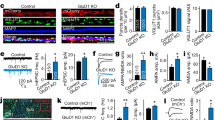
Abstract
Signaling through gap junctions (electrical synapses) is important in the development of the mammalian central nervous system. Abundant between neurons during postnatal development, gap junction coupling subsequently decreases and remains low in the adult, confined to specific subsets of neurons. Here we report that developmental uncoupling of gap junctions in the rat hypothalamus in vivo and in vitro is associated with a decrease in connexin 36 (Cx36) protein expression. Both developmental gap junction uncoupling and Cx36 downregulation are prevented by the blockade of NMDA glutamate receptors, action potentials and the calcium–cyclic AMP response element binding protein (CREB), and are accelerated by CREB overexpression. Developmental gap junction uncoupling and Cx36 downregulation are not affected by blockade of non-NMDA glutamate receptors, and do not occur in hypothalamic neurons from NMDA receptor subunit 1 (NMDAR1) knockout mice. These results demonstrate that NMDA receptor activity contributes to the developmental uncoupling of gap junctions via CREB-dependent downregulation of Cx36.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Becker, D.L. & Mobbs, P. Connexin alpha1 and cell proliferation in the developing chick retina. Exp. Neurol. 156, 326–332 (1999).
Bani-Yaghoub, M., Underhill, T.M. & Naus, C.C. Gap junction blockage interferes with neuronal and astroglial differentiation of mouse P19 embryonal carcinoma cells. Dev. Genet. 24, 69–81 (1999).
Lin, J.H. et al. Gap-junction-mediated propagation and amplification of cell injury. Nat. Neurosci. 1, 494–500 (1998).
Lo Turco, J.J. & Kriegstein, A.R. Clusters of coupled neuroblasts in embryonic neocortex. Science 252, 563–566 (1991).
Allen, F. & Warner, A. Gap junctional communication during neuromuscular junction formation. Neuron 6, 101–111 (1991).
Walton, K.D. & Navarrete, R. Postnatal changes in motoneurone electrotonic coupling studied in the in vitro rat lumbar spinal cord. J. Physiol. (Lond.) 433, 283–305 (1991).
Peinado, A., Yuste, R. & Katz, L.C. Gap junctional communication and the development of local circuits in neocortex. Cereb. Cortex 3, 488–498 (1993).
Personius, K., Chang, Q., Bittman, K., Panzer, J. & Balice-Gordon, R. Gap junctional communication among motor and other neurons shapes patterns of neural activity and synaptic connectivity during development. Cell. Commun. Adhes. 8, 329–333 (2001).
Roerig, B. & Feller, M.B. Neurotransmitters and gap junctions in developing neural circuits. Brain Res. Brain Res. Rev. 32, 86–114 (2000).
Bennett, M.V. & Zukin, R.S. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron 41, 495–511 (2004).
Naus, C.C. & Bani-Yaghoub, M. Gap junctional communication in the developing central nervous system. Cell Biol. Int. 22, 751–763 (1998).
Kandler, K. & Katz, L.C. Neuronal coupling and uncoupling in the developing nervous system. Curr. Opin. Neurobiol. 5, 98–105 (1995).
Kandler, K. & Katz, L.C. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J. Neurosci. 18, 1419–1427 (1998).
Meyer, T. Cell signaling by second messenger waves. Cell 64, 675–678 (1991).
Ben-Ari, Y. Developing networks play a similar melody. Trends Neurosci. 24, 353–360 (2001).
Garaschuk, O., Linn, J., Eilers, J. & Konnerth, A. Large-scale oscillatory calcium waves in the immature cortex. Nat. Neurosci. 3, 452–459 (2000).
Feller, M.B., Wellis, D.P., Stellwagen, D., Werblin, F.S. & Shatz, C.J. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science 272, 1182–1187 (1996).
Wong, R.O. Retinal waves and visual system development. Annu. Rev. Neurosci. 22, 29–47 (1999).
Milner, L.D. & Landmesser, L.T. Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. J. Neurosci. 19, 3007–3022 (1999).
Strata, F. et al. A pacemaker current in dye-coupled hilar interneurons contributes to the generation of giant GABAergic potentials in developing hippocampus. J. Neurosci. 17, 1435–1446 (1997).
Chang, Q., Gonzalez, M., Pinter, M.J. & Balice-Gordon, R.J. Gap junctional coupling and patterns of connexin expression among neonatal rat lumbar spinal motor neurons. J. Neurosci. 19, 10813–10828 (1999).
Mentis, G.Z., Diaz, E., Moran, L.B. & Navarrete, R. Increased incidence of gap junctional coupling between spinal motoneurones following transient blockade of NMDA receptors in neonatal rats. J. Physiol. (Lond.) 544, 757–764 (2002).
Connors, B.W., Benardo, L.S. & Prince, D.A. Coupling between neurons of the developing rat neocortex. J. Neurosci. 3, 773–782 (1983).
Peinado, A., Yuste, R. & Katz, L.C. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron 10, 103–114 (1993).
Venance, L., Glowinski, J. & Giaume, C. Electrical and chemical transmission between striatal GABAergic output neurones in rat brain slices. J. Physiol. (Lond.) 559, 215–230 (2004).
Kandler, K. & Katz, L.C. Relationship between dye coupling and spontaneous activity in developing ferret visual cortex. Dev. Neurosci. 20, 59–64 (1998).
Hatton, G.I. Synaptic modulation of neuronal coupling. Cell Biol. Int. 22, 765–780 (1998).
Rash, J.E. et al. Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin-36 but not connexin-32 or connexin-43. Proc. Natl. Acad. Sci. USA 97, 7573–7578 (2000).
Belluardo, N. et al. Expression of connexin36 in the adult and developing rat brain. Brain Res. 865, 121–138 (2000).
Long, M.A., Jutras, M.J., Connors, B.W. & Burwell, R.D. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat. Neurosci. 8, 61–66 (2005).
Srinivas, M. et al. Functional properties of channels formed by the neuronal gap junction protein connexin36. J. Neurosci. 19, 9848–9855 (1999).
Bessho, Y., Nawa, H. & Nakanishi, S. Selective up-regulation of an NMDA receptor subunit mRNA in cultured cerebellar granule cells by K(+)-induced depolarization and NMDA treatment. Neuron 12, 87–95 (1994).
Hardingham, G.E., Fukunaga, Y. & Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5, 405–414 (2002).
Greenberg, M.E. & Ziff, E.B. Signal transduction in the postsynaptic neuron: activity-dependent regulation of gene expression. in Synapses (eds. Cowan, M.W., Sudhof, T.C. & Stevens, C.F.) 357–391 (Johns Hopkins Univ. Press, Baltimore, 2001).
Lonze, B.E. & Ginty, D.D. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623 (2002).
Carlezon, W.A., Jr. et al. Regulation of cocaine reward by CREB. Science 282, 2272–2275 (1998).
Guzowski, J.F. & McGaugh, J.L. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc. Natl. Acad. Sci. USA 94, 2693–2698 (1997).
Venance, L. et al. Connexin expression in electrically coupled postnatal rat brain neurons. Proc. Natl. Acad. Sci. USA 97, 10260–10265 (2000).
Connors, B.W. & Long, M.A. Electrical synapses in the mammalian brain. Annu. Rev. Neurosci. 27, 393–418 (2004).
Galarreta, M. & Hestrin, S. Electrical synapses between GABA-releasing interneurons. Nat. Rev. Neurosci. 2, 425–433 (2001).
Xia, Z. & Storm, D.R. Regulatory Properties of the Mammalian Adenylyl Cyclases Ch. 7, 105–134 (R.G. Landes Austin, Texas, 1996).
Kornhuber, J. & Weller, M. Psychotogenicity and N-methyl-D-aspartate receptor antagonism: implications for neuroprotective pharmacotherapy. Biol. Psychiatry 41, 135–144 (1997).
Facchinetti, F. et al. Structural, neurochemical and behavioural consequences of neonatal blockade of NMDA receptor through chronic treatment with CGP 39551 or MK-801. Brain Res. Dev. Brain Res. 74, 219–224 (1993).
Belousov, A.B., Hunt, N.D., Raju, R.P. & Denisova, J.V. Calcium-dependent regulation of cholinergic cell phenotype in the hypothalamus in vitro. J. Neurophysiol. 88, 1352–1362 (2002).
Aberg, N.D., Ronnback, L. & Eriksson, P.S. Connexin43 mRNA and protein expression during postnatal development of defined brain regions. Brain Res. Dev. Brain Res. 115, 97–101 (1999).
Belousov, A.B. & van den Pol, A.N. Local synaptic release of glutamate from neurons in the rat hypothalamic arcuate nucleus. J. Physiol. (Lond.) 499, 747–761 (1997).
Belousov, A.B., O'Hara, B.F. & Denisova, J.V. Acetylcholine becomes the major excitatory neurotransmitter in the hypothalamus in vitro in the absence of glutamate excitation. J. Neurosci. 21, 2015–2027 (2001).
Luther, J.A. & Tasker, J.G. Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J. Physiol. (Lond.) 523, 193–209 (2000).
Li, Y., Erzurumlu, R.S., Chen, C., Jhaveri, S. & Tonegawa, S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell 76, 427–437 (1994).
Sugiura, N., Patel, R.G. & Corriveau, R.A. N-methyl-D-aspartate receptors regulate a group of transiently expressed genes in the developing brain. J. Biol. Chem. 276, 14257–14263 (2001).
Acknowledgements
We are grateful to J. Denisova, E. Leininger, I.R. Popescu and T. Stuart for technical contributions and to R.L. Neve (Harvard Medical School) for supplying CREB viral vectors. This research was supported by a Louisiana Board of Regents Research Competitiveness Subprogram award to R.A.C.; and by a US National Institutes of Health grant (RO1 DA015088-01A1), a National Science Foundation grant (IBN-0117603) and an American Heart Association grant (0350530N) to A.B.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Initial developmental increase in gap junction coupling and Cx36 expression in the hypothalamus in vivo and in vitro. (PDF 347 kb)
Supplementary Fig. 2
Effects of NMDA on cytoplasmic Ca2+ activity in hypothalamic cultures obtained from wild–type and NMDAR1 knockout mice. (PDF 227 kb)
Supplementary Fig. 3
Transient developmental increase in gap junction coupling and Cx36 expression are mediated by action potential–dependent mechanisms. (PDF 117 kb)
Rights and permissions
About this article
Cite this article
Arumugam, H., Liu, X., Colombo, P. et al. NMDA receptors regulate developmental gap junction uncoupling via CREB signaling. Nat Neurosci 8, 1720–1726 (2005). https://doi.org/10.1038/nn1588
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1588
This article is cited by
-
NMDA Receptors Contribute to Postnatal Developmental Downregulation of Connexin Expressions in the Rat Locus Coeruleus
Iranian Journal of Science (2023)
-
Step by step: cells with multiple functions in cortical circuit assembly
Nature Reviews Neuroscience (2022)
-
Excitatory synapses and gap junctions cooperate to improve Pv neuronal burst firing and cortical social cognition in Shank2-mutant mice
Nature Communications (2021)
-
Gap junctions and hemichannels: communicating cell death in neurodevelopment and disease
BMC Cell Biology (2017)
-
Phosphorylation of Connexin 43 by Cdk5 Modulates Neuronal Migration During Embryonic Brain Development
Molecular Neurobiology (2016)