Abstract
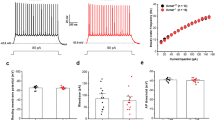
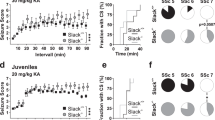
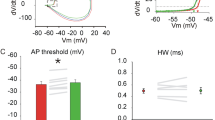
Synaptic inhibition within the hippocampus dentate gyrus serves a 'low-pass filtering' function that protects against hyperexcitability that leads to temporal lobe seizures. Here we demonstrate that calcium-activated potassium (BK) channel accessory β4 subunits serve as key regulators of intrinsic firing properties that contribute to the low-pass filtering function of dentate granule cells. Notably, a critical β4 subunit function is to preclude BK channels from contributing to membrane repolarization and thereby broaden action potentials. Longer-duration action potentials secondarily recruit SK channels, leading to greater spike frequency adaptation and reduced firing rates. In contrast, granule cells from β4 knockout mice show a gain-of-function for BK channels that sharpens action potentials and supports higher firing rates. Consistent with breakdown of the dentate filter, β4 knockouts show distinctive seizures emanating from the temporal cortex, demonstrating a unique nonsynaptic mechanism for gate control of hippocampal synchronization leading to temporal lobe epilepsy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sloviter, R.S. et al. “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J. Comp. Neurol. 459, 44–76 (2003).
Ratzliff, A.H., Santhakumar, V., Howard, A. & Soltesz, I. Mossy cells in epilepsy: rigor mortis or vigor mortis? Trends Neurosci. 25, 140–144 (2002).
Coulter, D.A. Mossy fiber zinc and temporal lobe epilepsy: pathological association with altered “epileptic” gamma-aminobutyric acid A receptors in dentate granule cells. Epilepsia 41 (Suppl.), S96–S99 (2000).
Buhl, E.H., Otis, T.S. & Mody, I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science 271, 369–373 (1996).
Garcia, M.L., Gao, Y., McManus, O.B. & Kaczorowski, G.J. Potassium channels: from scorpion venoms to high-resolution structure. Toxicon 39, 739–748 (2001).
Stocker, M. Ca(2+)-activated K(+) channels: molecular determinants and function of the SK family. Nat. Rev. Neurosci. 5, 758–770 (2004).
Stocker, M., Hirzel, K., D'Hoedt, D. & Pedarzani, P. Matching molecules to function: neuronal Ca2+-activated K+ channels and afterhyperpolarizations. Toxicon 43, 933–949 (2004).
Greffrath, W. et al. Contribution of Ca2+-activated K+ channels to hyperpolarizing after-potentials and discharge pattern in rat supraoptic neurones. J. Neuroendocrinol. 16, 577–588 (2004).
Engel, J., Schultens, H.A. & Schild, D. Small conductance potassium channels cause an activity-dependent spike frequency adaptation and make the transfer function of neurons logarithmic. Biophys. J. 76, 1310–1319 (1999).
Teshima, K., Kim, S.H. & Allen, C.N. Characterization of an apamin-sensitive potassium current in suprachiasmatic nucleus neurons. Neuroscience 120, 65–73 (2003).
Yen, J.C., Chan, J.Y. & Chan, S.H. Involvement of apamin-sensitive SK channels in spike frequency adaptation of neurons in nucleus tractus solitarii of the rat. J. Biomed. Sci. 6, 418–424 (1999).
Bond, C.T. et al. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J. Neurosci. 24, 5301–5306 (2004).
Blank, T., Nijholt, I., Kye, M.J. & Spiess, J. Small conductance Ca2+-activated K+ channels as targets of CNS drug development. Curr. Drug Targets CNS Neurol. Disord. 3, 161–167 (2004).
Faber, E.S. & Sah, P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J. Neurosci. 22, 1618–1628 (2002).
Cui, J., Cox, D.H. & Aldrich, R.W. Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca-activated K+ channels. J. Gen. Physiol. 109, 647–673 (1997).
Sanchez, M. & McManus, O.B. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35, 963–968 (1996).
Hu, H. et al. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J. Neurosci. 21, 9585–9597 (2001).
Swensen, A.M. & Bean, B.P. Ionic mechanisms of burst firing in dissociated Purkinje neurons. J. Neurosci. 23, 9650–9663 (2003).
Shao, L.R., Halvorsrud, R., Borg-Graham, L. & Storm, J.F. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J. Physiol. (Lond.) 521, 135–146 (1999).
Faber, E.S. & Sah, P. Ca2+-activated K+ (BK) channel inactivation contributes to spike broadening during repetitive firing in the rat lateral amygdala. J. Physiol. (Lond.) 552, 483–497 (2003).
Reinhart, P.H., Chung, S. & Levitan, I.B. A family of calcium-dependent potassium channels from rat brain. Neuron 2, 1031–1041 (1989).
Wang, G. & Lemos, J.R. Tetrandrine blocks a slow, large-conductance, Ca(2+)-activated potassium channel besides inhibiting a non-inactivating Ca2+ current in isolated nerve terminals of the rat neurohypophysis. Pflugers Arch. 421, 558–565 (1992).
Meera, P., Wallner, M. & Toro, L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc. Natl. Acad. Sci. USA 97, 5562–5567 (2000).
Lippiat, J.D., Standen, N.B., Harrow, I.D., Phillips, S.C. & Davies, N.W. Properties of BK(Ca) channels formed by bicistronic expression of hSloalpha and beta1–4 subunits in HEK293 cells. J. Membr. Biol. 192, 141–148 (2003).
Behrens, R. et al. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett. 474, 99–106 (2000).
Weiger, T.M. et al. A novel nervous system beta subunit that downregulates human large conductance calcium-dependent potassium channels. J. Neurosci. 20, 3563–3570 (2000).
Brenner, R., Jegla, T.J., Wickenden, A., Liu, Y. & Aldrich, R.W. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 275, 6453–6461 (2000).
Ha, T.S., Heo, M.S. & Park, C.S. Functional effects of auxiliary beta4-subunit on rat large-conductance Ca(2+)-activated K(+) channel. Biophys. J. 86, 2871–2882 (2004).
Sah, P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 19, 150–154 (1996).
Williamson, P.D. & Engel, J. in Epilepsy: a Comprehensive Textbook (eds. Engel, J. & Pedley, T.A.) 557–566 (Lippincott-Raven, Philadelphia, 1997).
Tian, L., Knaus, H.G. & Shipston, M.J. Glucocorticoid regulation of calcium-activated potassium channels mediated by serine/threonine protein phosphatase. J. Biol. Chem. 273, 13531–13536 (1998).
Bielefeldt, K. & Jackson, M.B. A calcium-activated potassium channel causes frequency-dependent action-potential failures in a mammalian nerve terminal. J. Neurophysiol. 70, 284–298 (1993).
Nadler, J.V. The recurrent mossy fiber pathway of the epileptic brain. Neurochem. Res. 28, 1649–1658 (2003).
Heinemann, U. et al. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res. Suppl. 7, 273–280 (1992).
Sah, P. & Faber, E.S. Channels underlying neuronal calcium-activated potassium currents. Prog. Neurobiol. 66, 345–353 (2002).
Rudy, B. & McBain, C.J. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 24, 517–526 (2001).
Lau, D. et al. Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J. Neurosci. 20, 9071–9085 (2000).
Juhng, K.N. et al. Induction of seizures by the potent K+ channel-blocking scorpion venom peptide toxins tityustoxin-K(alpha) and pandinustoxin-K(alpha). Epilepsy Res. 34, 177–186 (1999).
Jin, W., Sugaya, A., Tsuda, T., Ohguchi, H. & Sugaya, E. Relationship between large conductance calcium-activated potassium channel and bursting activity. Brain Res. 860, 21–28 (2000).
Du, W. et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat. Genet. 37, 733–738 (2005).
Kamal, A., Artola, A., Biessels, G.J., Gispen, W.H. & Ramakers, G.M. Increased spike broadening and slow afterhyperpolarization in CA1 pyramidal cells of streptozotocin-induced diabetic rats. Neuroscience 118, 577–583 (2003).
Mody, I., Kohr, G., Otis, T.S. & Staley, K.J. The electrophysiology of dentate gyrus granule cells in whole-cell recordings. Epilepsy Res. Suppl. 7, 159–168 (1992).
Henze, D.A., Wittner, L. & Buzsaki, G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat. Neurosci. 5, 790–795 (2002).
Mori, M., Abegg, M.H., Gahwiler, B.H. & Gerber, U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature 431, 453–456 (2004).
Jung, M.W. & McNaughton, B.L. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus 3, 165–182 (1993).
Kobayashi, M. & Buckmaster, P.S. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J. Neurosci. 23, 2440–2452 (2003).
Mott, D.D., Xie, C.W., Wilson, W.A., Swartzwelder, H.S. & Lewis, D.V. GABAB autoreceptors mediate activity-dependent disinhibition and enhance signal transmission in the dentate gyrus. J. Neurophysiol. 69, 674–691 (1993).
Strassle, B.W., Menegola, M., Rhodes, K.J. & Trimmer, J.S. Light and electron microscopic analysis of KChIP and Kv4 localization in rat cerebellar granule cells. J. Comp. Neurol. 484, 144–155 (2005).
Moyer, J.R., Jr . & Brown, T.H. Methods for whole-cell recording from visually preselected neurons of perirhinal cortex in brain slices from young and aging rats. J. Neurosci. Methods 86, 35–54 (1998).
Acknowledgements
We thank S. Wiler for technical assistance and B.S. Rothberg for critical reading of the manuscript. This work was supported by US National Institutes of Health grants NS29709 and HD24064 to J.L.N., American Heart Association grant 02250724 to Q.H.C. and a University of Texas Health Science Center Executive Research Committee grant to R.B. R.W.A. is an investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Tissue distribution of β4 gene expression. (PDF 195 kb)
Supplementary Fig. 2
Effect of SK channel block on firing properties during a 300 pA, 900 ms current injection utilizing UCL1684. (PDF 157 kb)
Supplementary Video 1
Spontaneous limbic seizure in BK β4−/− mouse. (MOV 2653 kb)
Rights and permissions
About this article
Cite this article
Brenner, R., Chen, Q., Vilaythong, A. et al. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci 8, 1752–1759 (2005). https://doi.org/10.1038/nn1573
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1573
This article is cited by
-
Peroxisome proliferator-activated receptor-α activation facilitates contextual fear extinction and modulates intrinsic excitability of dentate gyrus neurons
Translational Psychiatry (2023)
-
Functionally distinct POMC-expressing neuron subpopulations in hypothalamus revealed by intersectional targeting
Nature Neuroscience (2021)
-
A familiar study on self-limited childhood epilepsy patients using hIPSC-derived neurons shows a bias towards immaturity at the morphological, electrophysiological and gene expression levels
Stem Cell Research & Therapy (2021)
-
Upregulation of Beta4 subunit of BKCa channels in the anterior cingulate cortex contributes to mechanical allodynia associated anxiety-like behaviors
Molecular Brain (2020)
-
The molecular nature of the 17β-Estradiol binding site in the voltage- and Ca2+-activated K+ (BK) channel β1 subunit
Scientific Reports (2019)