Abstract
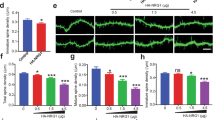
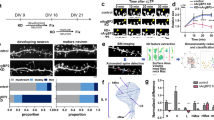
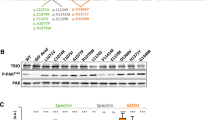
Of 11 genes involved in nonspecific X-linked mental retardation (MRX), three encode regulators or effectors of the Rho GTPases, suggesting an important role for Rho signaling in cognitive function. It remains unknown, however, how mutations in Rho-linked genes lead to MRX. Here we report that oligophrenin-1, a Rho-GTPase activating protein that is absent in a family affected with MRX, is required for dendritic spine morphogenesis. Using RNA interference and antisense RNA approaches, we show that knock-down of oligophrenin-1 levels in CA1 neurons in rat hippocampal slices significantly decreases spine length. This phenotype can be recapitulated using an activated form of RhoA and rescued by inhibiting Rho-kinase, indicating that reduced oligophrenin-1 levels affect spine length by increasing RhoA and Rho-kinase activities. We further demonstrate an interaction between oligophrenin-1 and the postsynaptic adaptor protein Homer. Our findings provide the first insight into how mutations in a Rho-linked MRX gene may compromise neuronal function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chelly, J. & Mandel, J.L. Monogenic causes of X-linked mental retardation. Nat. Rev. Genet. 2, 669–680 (2001).
Frints, S.G., Froyen, G., Marynen, P. & Fryns, J.P. X-linked mental retardation: vanishing boundaries between non-specific (MRX) and syndromic (MRXS) forms. Clin. Genet. 62, 423–432 (2002).
Ramakers, G.J. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci. 25, 191–199 (2002).
Van Aelst, L. & D'Souza-Schorey, C. Rho GTPases and network signaling. Genes Dev. 11, 2295–2322 (1997).
Luo, L. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1, 173–180 (2000).
Nakayama, A.Y., Harms, M.B. & Luo, L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J. Neurosci. 20, 5329–5338 (2000).
Tashiro, A., Minden, A. & Yuste, R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb. Cortex 10, 927–938 (2000).
Billuart, P. et al. Oligophrenin-1 encodes a rhoGAP protein involved in X-linked mental retardation. Nature 392, 923–926 (1998).
Allen, K.M. et al. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat. Genet. 20, 25–30 (1998).
Kutsche, K. et al. Mutations in ARHGEF6, encoding a guanine nucleotide exchange factor for Rho GTPases, in patients with X-linked mental retardation. Nat. Genet. 26, 247–250 (2000).
Fauchereau, F. et al. The RhoGAP activity of OPHN1, a new F-actin-binding protein is negatively controlled by its amino-terminal domain. Mol. Cell. Neurosci. 23, 574–586 (2003).
Hering, H. & Sheng, M. Dendritic spines: structure, dynamics and regulation. Nat. Rev. Neurosci. 2, 880–888 (2001).
Engert, F. & Bonhoeffer, T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70 (1999).
Maletic-Savatic, M., Malinow, R. & Svoboda, K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283, 1923–1927 (1999).
Toni, N., Buchs, P.A., Nikonenko, I., Bron, C.R. & Muller, D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402, 421–425 (1999).
Matsuzaki, M. et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 4, 1086–1092 (2001).
Comery, T.A., Stamoudis, C.X., Irwin, S.A. & Greenough, W.T. Increased density of multiple-head dendritic spines on medium-sized spiny neurons of the striatum in rats reared in a complex environment. Neurobiol. Learn. Mem. 66, 93–96 (1996).
Purpura, D.P. Dendritic spine “dysgenesis” and mental retardation. Science 186, 1126–1128 (1974).
Kaufmann, W.E. & Moser, H.W. Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex 10, 981–991 (2000).
Fiala, J.C., Spacek, J. & Harris, K.M. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res. Rev. 39, 29–54 (2002).
Harris, K.M. Structure, development, and plasticity of dendritic spines. Curr. Opin. Neurobiol. 9, 343–348 (1999).
Comery, T.A. et al. Abnormal dendritic spines in fragile X knockout mice: Maturation and pruning deficits. Proc. Natl. Acad. Sci. USA 94, 5401–5404 (1997).
Nimchinsky, E.A., Oberlander, A.M. & Svoboda, K. Abnormal development of dendritic spines in FMR1 knock-out mice. J. Neurosci. 21, 5139–5146 (2001).
Uehata, M. et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389, 990–994 (1997).
Amano, M., Fukata, Y. & Kaibuchi, K. Regulation and functions of Rho-associated kinase. Exp. Cell Res. 261, 44–51 (2000).
Beneken, J. et al. Structure of the Homer EVH1 domain-peptide complex reveals a new twist in polyproline recognition. Neuron 26, 143–154 (2000).
Fagni, L., Worley, P.F. & Ango, F. Homer as both a scaffold and transduction molecule. Sci. STKE 137, 1–7 (2002).
Xiao, B., Cheng, J. & Worley, P.F. Homer: a link between neural activity and glutamate receptor function. Curr. Opin. Neurobiol. 10, 370–374 (2000).
Huber, K.M., Gallagher, S.M., Warren, S.T. & Bear, M.F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. USA 99, 7746–7750 (2003).
Billuart, P., Winter, C.G., Maresh, A., Zhao, X. & Luo, L. Regulating axon branch stability: the role of p190 RhoGAP in repressing a retraction signaling pathway. Cell 107, 195–207 (2001).
Sala, C. et al. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron 31, 115–130 (2001).
Sala, C. et al. Inhibition of dendritic spine morphogenesis and synaptic transmission by activity-inducible protein Homer1a. J. Neurosci. 23, 6327–6337 (2003).
Fischer, M., Kaech, S., Wagner, U., Brinkhaus, H. & Matus, A. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat. Neurosci. 3, 887–894 (2000).
Star, E.N., Kwiatkowski, D.J. & Murthy, V.N. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat. Neurosci. 5, 239–246 (2002).
Ackermann, M. & Matus, A. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat. Neurosci. 6, 1194–1200 (2003).
McKinney, R.A., Capogna, M., Dürr, R., Gähwiler, B.H. & Thompson, S.M. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat. Neurosci. 2, 44–49 (1999).
Sin, W.C., Haas, K., Ruthazer, E.S. & Cline, H.T. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature 419, 475–480 (2002).
Yuan, J.P. et al. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 114, 777–789 (2003).
Korkotian, E. & Segal, M. Release of calcium from stores alters the morphology of dendritic spines in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA 96, 12068–12072 (1999).
Hayashi, K. et al. Modulatory role of Drebrin on the cytoskeleton within dendritic spines in the rat cerebral cortex. J. Neurosci. 16, 7161–7170 (1996).
Majewska, A., Tashiro, A. & Yuste, R. Regulation of spine calcium dynamics by rapid spine motility. J. Neurosci. 20, 8262–8268 (2000).
Yuste, R., Majewska, A. & Holthoff, K. From form to function: calcium compartmentalization in dendritic spines. Nat. Neurosci. 3, 653–659 (2000).
Bergmann, C. et al. Oligophrenin-1 (OPHN1) gene mutation causes X-linked mental retardation with epilepsy, rostral ventricular enlargement and cerebellar hypoplasia. Brain 126, 1537–1544 (2003).
Philip, N. et al. Mutations in the oligophrenin-1 gene (OPHN1) cause X linked congenital cerebellar hypoplasia. J. Med. Genet. 40, 441–446 (2003).
Joneson, T., McDonough, M., Bar-Sagi, D. & Van Aelst, L. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science 274, 1374–1376 (1996).
Pak, D.T., Yang, S., Rudolph-Correia, S., Kim, E. & Sheng, M. Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron 31, 289–303 (2001).
Stoppini, L., Buchs, P.A. & Muller, D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 37, 173–182 (1991).
Yamaguchi, Y., Katoh, H., Yasui, H., Mori, K. & Negishi, M. RhoA inhibits the nerve growth factor-induced Rac1 activation through Rho-associated kinase-dependent pathway. J. Biol. Chem. 276, 18977–18983 (2001).
Lau, L.F. et al. Interaction of the N-methyl-D-aspartate receptor complex with a novel synapse-associated protein, SAP102. J. Biol. Chem. 271, 21622–21628 (1996).
Cho, K.O., Hunt, C.A. & Kennedy, M.B. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron 9, 929–942 (1992).
Acknowledgements
We thank A. Piccini, E. Ruthazer, B. Burbach, C. Kopec, K. Jensen and H. Hsieh for technical assistance. We also thank H. Cline, R. Malinow, J. Skowronski, K. Svoboda, J. Rodriguez and members of the Van Aelst Laboratory for discussions and/or critical reading of the manuscript. This work was supported by the National Institutes of Health and Dana Foundation (to L.V.A.), the Wellcome Trust (to S.E.N. and C.J.A.) and the National Institutes of Health (to E.E.G).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Typical biolistic transfection and morphology of a hippocampal slice. The left panel is a fluorescence image to show the transfected GFP-expressing cells, while the right panel shows the morphology of a hippocampal slice. Indicated are the CA1, CA3, and dentate gyrus (DG) regions, as well as glial cells (glia). In the hippocampal slice, axons from CA3 pyramidal neurons synapse onto dendritic spines of CA1 pyramidal neurons. Three GFP expressing cells can be observed in the CA1 and CA3 regions; this is the average number of transfected cells in a typical imaging experiment. Only cells from the CA1 region were imaged and analyzed. The very low transfection rate in these slices (estimated 0.1%), and the very small number of synapses likely to be made on a transfected CA1 cell by a transfected CA3 cell in this system (< 1%), strongly suggest that the effect of reduced oligophrenin-1 levels on spine morphology are due to a post-synaptic role for oligophrenin-1. Scale bar, 250 µm. (JPG 44 kb)
Rights and permissions
About this article
Cite this article
Govek, EE., Newey, S., Akerman, C. et al. The X-linked mental retardation protein oligophrenin-1 is required for dendritic spine morphogenesis. Nat Neurosci 7, 364–372 (2004). https://doi.org/10.1038/nn1210
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1210
This article is cited by
-
Drosophila Graf regulates mushroom body β-axon extension and olfactory long-term memory
Molecular Brain (2021)
-
Spine impairment in mice high-expressing neuregulin 1 due to LIMK1 activation
Cell Death & Disease (2021)
-
Inhibition of RhoA Activity Does Not Rescue Synaptic Development Abnormalities and Long-Term Cognitive Impairment After Sevoflurane Exposure
Neurochemical Research (2021)
-
ARHGAP10, which encodes Rho GTPase-activating protein 10, is a novel gene for schizophrenia risk
Translational Psychiatry (2020)
-
Role of Per3, a circadian clock gene, in embryonic development of mouse cerebral cortex
Scientific Reports (2019)