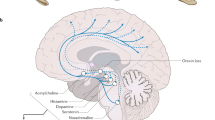
Abstract
Delineating the basic mechanisms that regulate sleep will likely result in the development of better treatments for sleep disorders. The hypothalamus is now recognized as a key center for sleep regulation, with hypothalamic neurotransmitter systems providing the framework for therapeutic advances. An increased awareness of the close interaction between sleep and homeostatic systems is also emerging. Progress has occurred in the understanding of narcolepsy—molecular techniques have identified the lateral hypothalamic hypocretin (orexin) neuropeptide system as key to the disorder. Other sleep disorders are now being tackled in the same way and are likely to yield to efforts combining basic and clinical research. Here we highlight the role of the hypothalamus in sleep physiology and discuss neurotransmitter systems, such as adenosine, dopamine, GABA, histamine and hypocretin, that may have therapeutic applications for sleep disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Rebecca Henretta
Similar content being viewed by others
References
Kryger, M., Roth, T. & Dement, W.C. Principles and Practice of Sleep Medicine 3rd edn. (WB Saunders Company, New York, 2000).
Van Economo, C. Sleep as a problem of localization. J. Nerv. Mental Disease 71, 249–269 (1931).
Bernardis, L.L. & Bellinger, L.L. The lateral hypothalamic area revisited: ingestive behavior. Neurosc. Behav. Rev. 20, 189–287 (1996).
Moruzzi, G. & Magoun, H.W. Brain stem reticular formation and activation of the EEG. Electroencephalogr. Clin. Neurophysiol. 1, 455–473 (1949).
Rechtschaffen, A., Bergmann, B.M., Everson, C.A., Kushida, C.A. & Gilliland, M.A. Sleep deprivation in the rat: X. Integration and discussion of the findings. Sleep 25, 68–87 (2002).
Spiegel, K., Leproult, R. & Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 354, 1435–1439 (1999).
Pace-Schott, E.F. & Hobson, J.A. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat. Rev. Neurosci. 3, 591–605 (2002).
Steriade, M. Arousal: revisiting the reticular activating system. Science 272, 225–226 (1996).
Wisor, J.P., Nishino, S., Sora, I., Uhl, G.H., Mignot, E. & Edgar, D.M. Dopaminergic role in stimulant-induced wakefulness. J. Neurosci. 21, 1787–1794 (2001).
Miller, J.D., Farber, J., Gatz, P., Roffwarg, H. & German, D.C. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res. 273, 133–141 (1983).
Rye, D.B. & Jankovic, J. Emerging views of dopamine in modulating sleep/wake state from an unlikely source: PD. Neurology 58, 341–346 (2002).
Lu, J., Xu, M. & Saper, C.B. Identification of wake-active dopaminergic neurons in the ventral periaqueducal gray. Sleep 25, supplement, A290 (2002).
Smith, T.A. Type A gamma-aminobutyric acid (GABAA) receptor subunits and benzodiazepine binding: significance to clinical syndromes and their treatment. Br. J. Biomed. Sci. 58, 111–121 (2001).
Crestani, F., Martin, J.R., Mohler, H. & Rudolph, U. Mechanism of action of the hypnotic zolpidem in vivo. Br. J. Pharmacol. 131, 1251–1254 (2000).
Tobler, I., Kopp, C., Deboer, T. & Rudolph, U. Diazepam-induced changes in sleep: role of the alpha 1 GABA(A) receptor subtype. Proc. Natl. Acad. Sci. USA 98, 6464–6469 (2001).
Bernasconi, R., Mathivet, P., Bischoff, S. & Marescaux, C. Gamma-hydroxybutyric acid: an endogenous neuromodulator with abuse potential? Trends Pharmacol. Sci. 20, 135–141 (1999).
Strecker, R.E. et al. Extracellular histamine levels in the feline preoptic/anterior hypothalamic area during natural sleep-wakefulness and prolonged wakefulness: an in vivo microdialysis study. Neuroscience 113, 663–670 (2002).
Parmentier, R., Ohtsu, H., Djebbara-Hannas, Z., Valatx, J.L., Watanabe, T. & Lin, J.S. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J. Neurosci. 22, 7695–7711 (2002).
Dugovic, C. et al. Sleep in mice lacking the histamine H3 receptor, a putative genetic animal model for REM behavior disorder. Sleep 25, supplement, A114 (2002).
Beuckmann, C.T. & Yanagisawa, M. Orexins: from neuropeptides to energy homeostasis and sleep/wake regulation. J. Mol. Med. 80, 329–342 (2002).
Taheri, S., Zeitzer, J.M. & Mignot, E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu. Rev. Neurosci. 25, 283–313 (2002).
Marcus, J.N. et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 435, 6–25 (2001).
Yoshida, Y. et al. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur. J. Neurosci. 14, 1075–1081 (2001).
Kiyashchenko, L.I. et al. Release of hypocretin (orexin) during waking and sleep states. J. Neurosci. 22, 5282–5286 (2002).
Yamanaka, A. et al. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem. Biophys. Res. Commun. 290, 1237–1245 (2002).
Saper, C.B., Chou, T.C. & Scammell, T.E. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 24, 726–731 (2001).
Suntsova, N., Szymusiak, R., Alam, M.N., Guzman-Marin, R. & McGinty, D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J. Physiol. 543, 665–677 (2002).
Dijk, D.J. & Czeisler, C.A. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosc. Lett. 166, 63–68 (1994).
Edgar, D.M., Dement, W.C. & Fuller, C.A. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J. Neurosci. 13, 1065–1079 (1993).
Wager-Smith, K. & Kay, S.A. Circadian rhythm genetics: from flies to mice to humans. Nat. Genet. 26, 23–27 (2000).
Kramer, A. et al. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science 294, 2511–2515 (2001).
Cheng, M.Y. et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417, 405–410 (2002).
Shaw, P.J., Tononi, G., Greenspan, R.J. & Robinson, D.F. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature 417, 287–291 (2002).
Naylor, E. et al. The circadian clock mutation alters sleep homeostasis in the mouse. J. Neurosci. 20, 8138–8143 (2000).
Porkka-Heiskanen, T., Strecker, R.E., Thakkar, M., Bjorkum, A.A., Greene, R.W. & McCarley, R.W. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science 276, 1265–1268 (1997).
Kubin, L., Tojima, H., Reignier, C., Pack, A.I. & Davies, R.O. Interaction of serotonergic excitatory drive to hypoglossal motoneurons with carbachol-induced, REM sleep-like atonia. Sleep 19, 187–195 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mignot, E., Taheri, S. & Nishino, S. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci 5 (Suppl 11), 1071–1075 (2002). https://doi.org/10.1038/nn944
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn944
This article is cited by
-
Improving Sleep to Improve Stress Resilience
Current Sleep Medicine Reports (2024)
-
The effect of transcranial direct current stimulation on sleep quality, resilience, and optimism
Current Psychology (2023)
-
The relationship between sleep and opioids in chronic pain patients
Journal of Behavioral Medicine (2021)
-
SAGE-217, A Novel GABAA Receptor Positive Allosteric Modulator: Clinical Pharmacology and Tolerability in Randomized Phase I Dose-Finding Studies
Clinical Pharmacokinetics (2020)