Abstract
The chromatin remodeling gene CHD8 represents a central node in neurodevelopmental gene networks implicated in autism. We examined the impact of germline heterozygous frameshift Chd8 mutation on neurodevelopment in mice. Chd8+/del5 mice displayed normal social interactions with no repetitive behaviors but exhibited cognitive impairment correlated with increased regional brain volume, validating that phenotypes of Chd8+/del5 mice overlap pathology reported in humans with CHD8 mutations. We applied network analysis to characterize neurodevelopmental gene expression, revealing widespread transcriptional changes in Chd8+/del5 mice across pathways disrupted in neurodevelopmental disorders, including neurogenesis, synaptic processes and neuroimmune signaling. We identified a co-expression module with peak expression in early brain development featuring dysregulation of RNA processing, chromatin remodeling and cell-cycle genes enriched for promoter binding by Chd8, and we validated increased neuronal proliferation and developmental splicing perturbation in Chd8+/del5 mice. This integrative analysis offers an initial picture of the consequences of Chd8 haploinsufficiency for brain development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Ho, L. & Crabtree, G.R. Chromatin remodelling during development. Nature 463, 474–484 (2010).
Chen, T. & Dent, S.Y.R. Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat. Rev. Genet. 15, 93–106 (2014).
Ronan, J.L., Wu, W. & Crabtree, G.R. From neural development to cognition: unexpected roles for chromatin. Nat. Rev. Genet. 14, 347–359 (2013).
Sanders, S.J. First glimpses of the neurobiology of autism spectrum disorder. Curr. Opin. Genet. Dev. 33, 80–92 (2015).
McCarthy, S.E. et al. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol. Psychiatry 19, 652–658 (2014).
Vissers, L.E.L.M., Gilissen, C. & Veltman, J.A. Genetic studies in intellectual disability and related disorders. Nat. Rev. Genet. 17, 9–18 (2016).
De Rubeis, S. et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215 (2014).
Iossifov, I. et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221 (2014).
Bernier, R. et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 158, 263–276 (2014).
Nishiyama, M. et al. Early embryonic death in mice lacking the beta-catenin-binding protein Duplin. Mol. Cell. Biol. 24, 8386–8394 (2004).
Sugathan, A. et al. CHD8 regulates neurodevelopmental pathways associated with autism spectrum disorder in neural progenitors. Proc. Natl. Acad. Sci. USA 111, E4468–E4477 (2014).
Cotney, J. et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat. Commun. 6, 6404 (2015).
Katayama, Y. et al. CHD8 haploinsufficiency results in autistic-like phenotypes in mice. Nature 537, 675–679 (2016).
Platt, R.J. et al. Chd8 Mutation Leads to autistic-like behaviors and impaired striatal circuits. Cell Rep. 19, 335–350 (2017).
Durak, O. et al. Chd8 mediates cortical neurogenesis via transcriptional regulation of cell cycle and Wnt signaling. Nat. Neurosci. 19, 1477–1488 (2016).
Silverman, J.L., Yang, M., Lord, C. & Crawley, J.N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 11, 490–502 (2010).
Silverman, J.L., Babineau, B.A., Oliver, C.F., Karras, M.N. & Crawley, J.N. Influence of stimulant-induced hyperactivity on social approach in the BTBR mouse model of autism. Neuropharmacology 68, 210–222 (2013).
Croft, D. et al. The Reactome pathway knowledgebase. Nucleic Acids Res. 42, D472–D477 (2014).
Sanders, S.J. et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron 87, 1215–1233 (2015).
Hormozdiari, F., Penn, O., Borenstein, E. & Eichler, E.E. The discovery of integrated gene networks for autism and related disorders. Genome Res. 25, 142–154 (2015).
Willsey, A.J. et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 155, 997–1007 (2013).
Darnell, J.C. et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011).
Voineagu, I. et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474, 380–384 (2011).
Parikshak, N.N. et al. Genome-wide changes in lncRNA, splicing, and regional gene expression patterns in autism. Nature 540, 423–427 (2016).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008).
Parikshak, N.N. et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 155, 1008–1021 (2013).
Jensen, L.J. et al. STRING 8--a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 37, D412–D416 (2009).
Alrahbeni, T. et al. Full UPF3B function is critical for neuronal differentiation of neural stem cells. Mol. Brain 8, 33 (2015).
Laumonnier, F. et al. Mutations of the UPF3B gene, which encodes a protein widely expressed in neurons, are associated with nonspecific mental retardation with or without autism. Mol. Psychiatry 15, 767–776 (2010).
Katz, Y., Wang, E.T., Airoldi, E.M. & Burge, C.B. Analysis and design of RNA sequencing experiments for identifying isoform regulation. Nat. Methods 7, 1009–1015 (2010).
Zhang, X. et al. Cell-type-specific alternative splicing governs cell fate in the developing cerebral cortex. Cell 166, 1147–1162.e15 (2016).
Tipps, M.E., Raybuck, J.D., Buck, K.J. & Lattal, K.M. Delay and trace fear conditioning in C57BL/6 and DBA/2 mice: issues of measurement and performance. Learn. Mem. 21, 380–393 (2014).
Brunner, D. et al. Comprehensive analysis of the 16p11.2 deletion and null Cntnap2 mouse models of autism spectrum disorder. PLoS One 10, e0134572 (2015).
Ellegood, J. et al. Clustering autism: using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Mol. Psychiatry 20, 118–125 (2015).
Etherton, M.R., Blaiss, C.A., Powell, C.M. & Südhof, T.C. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc. Natl. Acad. Sci. USA 106, 17998–18003 (2009).
Orosco, L.A. et al. Loss of Wdfy3 in mice alters cerebral cortical neurogenesis reflecting aspects of the autism pathology. Nat. Commun. 5, 4692 (2014).
Hagerman, R., Au, J. & Hagerman, P. FMR1 premutation and full mutation molecular mechanisms related to autism. J. Neurodev. Disord. 3, 211–224 (2011).
Lee, J.-A. et al. Cytoplasmic Rbfox1 regulates the expression of synaptic and autism-related genes. Neuron 89, 113–128 (2016).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Du, C., Ma, X., Meruvu, S., Hugendubler, L. & Mueller, E. The adipogenic transcriptional cofactor ZNF638 interacts with splicing regulators and influences alternative splicing. J. Lipid Res. 55, 1886–1896 (2014).
Yoo, S.-W., Motari, M.G. & Schnaar, R.L. Agenesis of the corpus callosum in Nogo receptor deficient mice. J. Comp. Neurol. 525, 291–301 (2017).
Yeung, J., Ha, T.J., Swanson, D.J. & Goldowitz, D. A novel and multivalent role of Pax6 in cerebellar development. J. Neurosci. 36, 9057–9069 (2016).
Nguyen, K.H., Yao, X.-H., Erickson, A.G., Mishra, S. & Nyomba, B.L.G. Glucose intolerance in aging male IGFBP-3 transgenic mice: differential effects of human IGFBP-3 and its mutant IGFBP-3 devoid of IGF binding ability. Endocrinology 156, 462–474 (2015).
Galazo, M.J., Emsley, J.G. & Macklis, J.D. Corticothalamic projection neuron development beyond subtype specification: Fog2 and intersectional controls regulate intraclass neuronal diversity. Neuron 91, 90–106 (2016).
Espuny-Camacho, I. et al. Hallmarks of Alzheimer's disease in stem-cell-derived human neurons transplanted into mouse brain. Neuron 93, 1066–1081.e8 (2017).
Huang, Z., Yu, Y., Shimoda, Y., Watanabe, K. & Liu, Y. Loss of neural recognition molecule NB-3 delays the normal projection and terminal branching of developing corticospinal tract axons in the mouse. J. Comp. Neurol. 520, 1227–1245 (2012).
Lloyd-Burton, S. & Roskams, A.J. SPARC-like 1 (SC1) is a diversely expressed and developmentally regulated matricellular protein that does not compensate for the absence of SPARC in the CNS. J. Comp. Neurol. 520, 2575–2590 (2012).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Silverman, J.L. et al. GABAB receptor agonist R-baclofen reverses social deficits and reduces repetitive behavior in two mouse models of autism. Neuropsychopharmacology 40, 2228–2239 (2015).
Flannery, B.M. et al. Behavioral assessment of NIH Swiss mice acutely intoxicated with tetramethylenedisulfotetramine. Neurotoxicol. Teratol. 47, 36–45 (2015).
Silverman, J.L. et al. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci. Transl. Med. 4, 131ra51 (2012).
Silverman, J.L. et al. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience 171, 1197–1208 (2010).
Wöhr, M. & Scattoni, M.L. Behavioural methods used in rodent models of autism spectrum disorders: current standards and new developments. Behav. Brain Res. 251, 5–17 (2013).
Chadman, K.K. et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 1, 147–158 (2008).
Rogers, D.C. et al. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm. Genome 8, 711–713 (1997).
Yang, M., Lewis, F.C., Sarvi, M.S., Foley, G.M. & Crawley, J.N. 16p11.2 Deletion mice display cognitive deficits in touchscreen learning and novelty recognition tasks. Learn Mem. 22, 622–632 (2015).
Thomas, A.M., Bui, N., Perkins, J.R., Yuva-Paylor, L.A. & Paylor, R. Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome. Psychopharmacology (Berl.) 219, 47–58 (2012).
Thomas, A. et al. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl.) 204, 361–373 (2009).
Henderson, C. et al. Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABAB receptors with arbaclofen. Sci. Transl. Med. 4, 152ra128 (2012).
Yang, M., Silverman, J.L. & Crawley, J.N. Automated three-chambered social approach task for mice. in Current Protocols in Neuroscience (eds. Gerfen, C.R., Holmes, A., Sibley, D., Skolnick, P. & Wray, S.) Chapter 8, Unit 8.26–8.26.16 (Wiley, 2011).
Scattoni, M.L., Ricceri, L. & Crawley, J.N. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 10, 44–56 (2011).
Bales, K.L. et al. Long-term exposure to intranasal oxytocin in a mouse autism model. Transl. Psychiatry 4, e480 (2014).
Skrzypiec-Spring, M., Grotthus, B., Szelag, A. & Schulz, R. Isolated heart perfusion according to Langendorff---still viable in the new millennium. J. Pharmacol. Toxicol. Methods 55, 113–126 (2007).
Cahill, L.S. et al. Preparation of fixed mouse brains for MRI. Neuroimage 60, 933–939 (2012).
Bock, N.A. et al. In vivo magnetic resonance imaging and semiautomated image analysis extend the brain phenotype for cdf/cdf mice. J. Neurosci. 26, 4455–4459 (2006).
Nieman, B.J. et al. Fast spin-echo for multiple mouse magnetic resonance phenotyping. Magn. Reson. Med. 54, 532–537 (2005).
Jones, D.K., Horsfield, M.A. & Simmons, A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn. Reson. Med. 42, 515–525 (1999).
Dorr, A.E., Lerch, J.P., Spring, S., Kabani, N. & Henkelman, R.M. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 42, 60–69 (2008).
Collins, D.L., Neelin, P., Peters, T.M. & Evans, A.C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 18, 192–205 (1994).
Avants, B.B. et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044 (2011).
Avants, B.B., Epstein, C.L., Grossman, M. & Gee, J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008).
Lerch, J.P. et al. Cortical thickness measured from MRI in the YAC128 mouse model of Huntington's disease. Neuroimage 41, 243–251 (2008).
Nieman, B.J., Flenniken, A.M., Adamson, S.L., Henkelman, R.M. & Sled, J.G. Anatomical phenotyping in the brain and skull of a mutant mouse by magnetic resonance imaging and computed tomography. Physiol. Genomics 24, 154–162 (2006).
Steadman, P.E. et al. Genetic effects on cerebellar structure across mouse models of autism using a magnetic resonance imaging atlas. Autism Res. 7, 124–137 (2014).
Ullmann, J.F.P., Janke, A.L., Reutens, D. & Watson, C. Development of MRI-based atlases of non-human brains. J. Comp. Neurol. 523, 391–405 (2015).
Genovese, C.R., Lazar, N.A. & Nichols, T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15, 870–878 (2002).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G.K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Wang, L., Wang, S. & Li, W. RSeQC: quality control of RNA-seq experiments. Bioinformatics 28, 2184–2185 (2012).
Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Robinson, M.D., McCarthy, D.J. & Smyth, G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Nord, A.S. et al. Rapid and pervasive changes in genome-wide enhancer usage during mammalian development. Cell 155, 1521–1531 (2013).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Feng, J., Liu, T. & Zhang, Y. Using MACS to identify peaks from ChIP-seq data. in Current Protocols in Bioinformatics (eds. Bateman, A., Draghici, S., Khurana, E, Orchard, S. & Pearson, W.R.) Chapter 2, Unit 2.14 (Wiley, 2011).
Chen, E.Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128 (2013).
Medina-Rivera, A. et al. RSAT 2015: regulatory sequence analysis tools. Nucleic Acids Res. 43, W50-6 (2015).
Zhang, B. & Horvath, S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 4, e17 (2005).
Van Laere, S. et al. Distinct molecular phenotype of inflammatory breast cancer compared to non-inflammatory breast cancer using Affymetrix-based genome-wide gene-expression analysis. Br. J. Cancer 97, 1165–1174 (2007).
Young, M.D., Wakefield, M.J., Smyth, G.K. & Oshlack, A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11, R14 (2010).
Rossin, E.J. et al. Proteins encoded in genomic regions associated with immune-mediated disease physically interact and suggest underlying biology. PLoS Genet. 7, e1001273 (2011).
Acknowledgements
Sequencing was performed at the UC Berkeley and UC Davis DNA cores. This work was supported by institutional funds from the UC Davis Center for Neuroscience, by the UC Davis MIND Institute Intellectual and Developmental Disabilities Research Center (U54 HD079125) and by NIGMS R35 GM119831. L.S.-F. was supported by the UC Davis Floyd and Mary Schwall Fellowship in Medical Research and by grant number T32-GM008799 from NIGMS-NIH. A.A.W. was supported by Training Grant number T32-GM007377 from NIH-NIGMS. R.C.-P. was supported by a Science Without Borders Fellowship from CNPq (Brazil). A.V., L.A.P. and D.E.D. were supported by National Institutes of Health grants R24HL123879, U01DE024427, R01HG003988, U54HG006997 and UM1HL098166. Research conducted at the E.O. Lawrence Berkeley National Laboratory was performed under Department of Energy Contract DE-AC02-05CH11231, University of California. J.E. and J.P.L. were supported by the Canadian Institute for Health Research, Brain Canada and the Ontario Brain Institute.
Author information
Authors and Affiliations
Contributions
A.L.G., L.S.-F., J.E., N.A.C. and M.A.R. are listed as joint first authors, as each led components of the experiments and analysis. A.L.G., L.S.-F., J.E., M.A.R., N.A.C., J.P.L., J.N.C., J.L.S., K.S.Z. and A.S.N. designed the experiments. Generation of mouse model: A.S.N., D.E.D., A.V., L.A.P., B.J.M., I.P.-F. and V.A.; mouse behavior: N.A.C., M.C.P., M.D.S., J.N.C. and J.L.S.; mouse MRI: J.E. and J.P.L.; genomics and molecular genetics: L.S.-F., A.L.G., I.Z., A.A.W., R.C.-P., S.L., B.J.M. and A.S.N.; neuroanatomy: A.L.G., M.A.R., T.W.S., I.Z., G.K., K.S.Z. A.L.G., L.S.-F., J.E., N.A.C., M.A.R., K.S.Z., J.N.C., J.L.S. and A.S.N. drafted the manuscript. All authors contributed to manuscript revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Raw western blots.
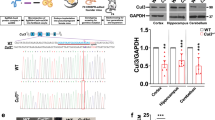
Western blots of Chd8 and Gapdh protein in adult 5-bp del and 14-bp del Chd8 mutant lines show decreased protein levels in Chd8+/del5 mice from both alleles. WT n = 3 for 280 kDa, WT n = 4 for 110 kDa, 5-bp n = 3, 14-bp n = 3. There is a significant difference in Chd8 protein abundance in Chd8+/del5 mice at 280 kDa (ANOVA *P = 0.0118, 5-bp *P = 0.0319; 14-bp *P = 0.0328) but not at 110 kDa (ANOVA P = 0.1676, 5-bp P = 0.1086, 14-bp P = 0.4509). B. Chd8 and Gapdh protein expression in WT and Chd8+/del5 (HT) mice at P0. There is a significant difference in Chd8 protein abundance in Chd8+/del5 mice at 280 kDa (n: WT = 9, Chd8+/del5 = 9; *P = 0.0089) and 110 kDa (**P = 0.001). C. Chd8 and Gapdh protein expression in Chd8+/del5 (HT) and WT mice at E14.5. There is a significant difference in Chd8 protein abundance in Chd8+/del5 mice at 280 kDa (n: WT = 6, Chd8+/del5 = 6; *P = 0.02) but not at 110 kDa (P = 0.9903). D. Raw Hnrnpa2b1 western blot from P0 forebrain. One-way ANOVA followed by unpaired post hoc t-tests used in A. Unpaired t-tests used for B-C. Error bars represent mean ± s.e.m.
Supplementary Figure 2 Chd8+/del5 mice from the replication cohort exhibit cognitive deficits, but no ASD social or repetitive phenotypes.
Chd8+/del5 mice exhibit deficits in learning and memory, including reduced freezing after fear conditioning in both context (A; t(1, 40) = 1.6607, P = 0.1046) and cued assays (B; t(1, 40) = 1.9593, P = 0.0571). Novel Object: Chd8+/del5 mice fail to show significant difference in exploration between a novel and familiarized object (C; WT F(1, 20) = 4.5583, P = 0.0453; Chd8+/del5 F(1, 19) = 0.7921, P = 0.3846). Social Approach: Chd8+/del5 mice do not exhibit differences relative to WT littermates in time spent in chamber with novel mouse (D; WT: F(1, 20) = 10.438, P = 0.0042; Chd8+/del5: F(1, 20) = 15.470, P = 0.0008), time sniffing a novel mouse (E; WT: F(1, 20) = 20.3750, P = 0.0002; Chd8+/del5: F(1, 20) = 30.3946, P = 0.00002), or chamber entries (F; F(1, 40) = 0.307, P = 0.583). Male-Female Social Interactions: Chd8+/del5 mice exhibit no differences between WT littermates in time spent engaged in nose to nose sniffs (G; t(1, 20) = 0.1520, P = 0.8807), nose to anogenital sniffs (H; t(1, 20) = 0.2492, P = 0.8057), time following (I; t(1, 20) = 1.6396, P = 0.1167), or ultrasonic vocalizations (J; t(1, 20) = 0.7010, P = 0.4914) with an estrus female. Repetitive Behavior: Chd8+/del5 mice do not exhibit any differences in time spent self-grooming (K; t(1, 40) = 0.2489, P = 0.8047). Open Field: Chd8+/del5 mice do not exhibit any differences in distance traveled (L; t(1, 40) = 0.9799, P = 0.3328). All data shown from 2nd cohort. Male mice were used in G-J; males and females were used in all other panels. Unpaired t-tests used for A-B, G-L; within genotype repeated measures ANOVA used for C-E; one-way ANOVA used for F. Error bars represent mean ± s.e.m.
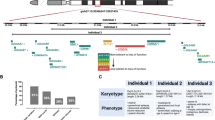
Supplementary Figure 3 Fractional anisotropy (FA) differences in several coronal slices throughout the brain.
Highlighted are FA differences measured as effect size between the Chd8+/del5 brains and corresponding WT brains thresholded at 1.0. Of interest is the lateral decrease and corresponding medial increase in FA surrounding the cerebral peduncle. It should be noted that these differences are only indicative of trends as no significant differences were found in our FA measures.
Supplementary Figure 4 qRT-PCR validation of differential mRNA expression at P0.
A-J. Data from 7 WT and 5 Chd8+/del5 (HT) P0 forebrains (with the exception of G, which used 7 Chd8+/del5 P0 forebrains), with reactions performed in triplicate. For each target, the one highest and one lowest values were removed from analysis to reduce noise. P-values from Student’s t-test. Primers reported in Table S4. A-J WT n: 6, 4, 6, 6, 6, 6, 7, 6, 6, 6; Chd8+/del5 n: 7, 4, 5, 5, 4, 5, 7, 5, 5, 4.
Supplementary Figure 5 Chd8 RNA-seq and ChIP-seq analyses.
A. Results from RSAT de novo motif analysis on our Chd8 ChIP-seq peaks. Highlighted are three representative results for significant YY1, NRF1, and NFYB motifs (left), and corresponding predicted motif sites within Chd8 ChIP-seq peaks (middle) and the identified motif (right). B-C. Comparison of DE genes in our RNA-seq data with two recent Chd8 models, Katayama et al. (2016) (germline heterozygous Chd8), and Durak et al. (2016) (Chd8 knockdown). Dots represent genes passing FDR < 0.05 in our data and in either Katayama et al. (2016) or Durak et al. (2016) compared to our model (n = 37 and 77, respectively). The red dot indicates Chd8. DE genes in our data are significantly enriched in both datasets, but overlap between our data is stronger with Durak et al. (2016) than with Katayama et al. (2016). D. Differential expression of example synaptic genes. Top panels show expression (log2RPKM) across stages, bottom shows first four early developmental stages only. From left to right: Scn2b, Cacna1e, Cacna2d1, Cacna1b. Solid line = mean, dashed line = ±1 s.e.m. Black: WT; Red: Chd8+/del5.
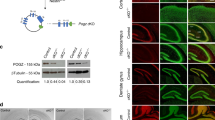
Supplementary Figure 6 P0 and P7 coronal sections reveal no obvious perturbations to cortical lamination.
Single optical sections of wild type (A-D, I-M) and Chd8+/del5 (E-H, N-R) brains were imaged at 4X and paired based on corresponding anatomical depth of section. Immunofluorescence corresponding to Ctip2 (green; B, F, J, O), Tbr1 (red; C, G, K, P), and Brn2 (magenta; L, Q) was collected via sequential imaging and merged with DAPI (blue; D, H, M, R). Scale bars in E and N are 500 μm. P0: Tbr1 thickness P = 0.34, Ctip2 thickness P = 0.81, P7: Tbr1 thickness P = 0.88, Ctip2 thickness P = 0.69, Brn2 thickness P = 0.32. P-values derived from Student’s t-test. P0: WT n = 8, Chd8+/del5 n = 10; P7: WT n = 7, Chd8+/del5 n = 10.
Supplementary Figure 7 Changes in splicing in Chd8+/del5 mice.
A. DE down-regulated genes are enriched in DS genes, but not up-regulated DE genes. DS genes from our data overlap significantly with DS genes identified in a recent postmortem ASD cortex study25. B. (Left) Genome Browser representation of the alternatively spliced Srsf7 intron (chr17:80,604,601-80,603,728). (Right) Isoform-specific qRT-PCR analysis of the Srsf7 intron across development in WT and e17.5 Chd8+/del5 (HT) mice. n: e12.5 = 7, e14.5 = 9, e17.5 Chd8+/del5 = 7, e17.5 WT = 7, P0 = 11. Student’s t-test, e14.5 WT to e17.5 Chd8+/del5, P = 0.30, e17.5 Chd8+/del5 to e17.5 WT, P = 0.62. Linear regression across developmental time points, adjusted R2 = 0.37, P = 1.32E-05. Error bars represent mean ± s.e.m.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1819 kb)
Supplementary Table 1
Summary of MRI volume by regions (XLSX 61 kb)
Supplementary Table 2
RNA sample and library data (XLSX 45 kb)
Supplementary Table 3
Differential expression summary table (XLSX 12110 kb)
Supplementary Table 4
Primers used for qPCR experiments (XLSX 47 kb)
Supplementary Table 5
Summary of GO enrichment (XLSX 94 kb)
Supplementary Table 6
Enrichment summary for Reactome pathways (XLSX 285 kb)
Supplementary Table 7
WGCNA results (XLSX 48 kb)
Supplementary Table 8
MISO results (XLSX 2669 kb)
Supplementary Software
Analysis scripts (ZIP 8 kb)
Rights and permissions
About this article
Cite this article
Gompers, A., Su-Feher, L., Ellegood, J. et al. Germline Chd8 haploinsufficiency alters brain development in mouse. Nat Neurosci 20, 1062–1073 (2017). https://doi.org/10.1038/nn.4592
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4592
This article is cited by
-
Current and future applications of light-sheet imaging for identifying molecular and developmental processes in autism spectrum disorders
Molecular Psychiatry (2024)
-
The complex etiology of autism spectrum disorder due to missense mutations of CHD8
Molecular Psychiatry (2024)
-
Small molecule modulators of chromatin remodeling: from neurodevelopment to neurodegeneration
Cell & Bioscience (2023)
-
Deletion of the autism-related gene Chd8 alters activity-dependent transcriptional responses in mouse postmitotic neurons
Communications Biology (2023)
-
CHD8 mutations increase gliogenesis to enlarge brain size in the nonhuman primate
Cell Discovery (2023)