Abstract
A sparse population of a few hundred primarily hypothalamic neurons forms the hub of a complex neuroglial network that controls reproduction in mammals by secreting the 'master molecule' gonadotropin-releasing hormone (GnRH). Timely postnatal changes in GnRH expression are essential for puberty and adult fertility. Here we report that a multilayered microRNA-operated switch with built-in feedback governs increased GnRH expression during the infantile-to-juvenile transition and that impairing microRNA synthesis in GnRH neurons leads to hypogonadotropic hypogonadism and infertility in mice. Two essential components of this switch, miR-200 and miR-155, respectively regulate Zeb1, a repressor of Gnrh transcriptional activators and Gnrh itself, and Cebpb, a nitric oxide–mediated repressor of Gnrh that acts both directly and through Zeb1, in GnRH neurons. This alteration in the delicate balance between inductive and repressive signals induces the normal GnRH-fuelled run-up to correct puberty initiation, and interfering with this process disrupts the neuroendocrine control of reproduction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
03 June 2016
In the version of this article initially published, the Figure 1e,f legend read, "Circulating levels of LH (left panels) and FSH (right panels) in GnRH cells of control (blue) and Dicer mutants (red)"; as the hormones were not measured in GhRH cells, it should have simply read "Circulating levels of LH (left panels) and FSH (right panels) in control (blue) and Dicer mutants (red)." Figure 2b was missing scale bars and has been replaced. The label "TSB-200" was missing from the rightmost bar in Figure 4d. And the treatment in Figure 5c was misidentified as TSB-200 instead of TSB-155. The errors have been corrected in the HTML and PDF versions of the article.
References
Ojeda, S.R. & Lomniczi, A. Puberty in 2013: unraveling the mystery of puberty. Nat. Rev. Endocrinol. 10, 67–69 (2014).
Schwanzel-Fukuda, M., Bick, D. & Pfaff, D.W. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res. Mol. Brain Res. 6, 311–326 (1989).
Mason, A.J. et al. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the HPG mouse. Science 234, 1366–1371 (1986).
de Roux, N. et al. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N. Engl. J. Med. 337, 1597–1602 (1997).
Harfe, B.D., McManus, M.T., Mansfield, J.H., Hornstein, E. & Tabin, C.J. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc. Natl. Acad. Sci. USA 102, 10898–10903 (2005).
Hasuwa, H., Ueda, J., Ikawa, M. & Okabe, M. miR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science 341, 71–73 (2013).
Papaioannou, M.D. & Nef, S. microRNAs in the testis: building up male fertility. J. Androl. 31, 26–33 (2010).
Bernstein, E., Caudy, A.A., Hammond, S.M. & Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366 (2001).
Yoon, H., Enquist, L.W. & Dulac, C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123, 669–682 (2005).
d'Anglemont de Tassigny, X., Ackroyd, K.J., Chatzidaki, E.E. & Colledge, W.H. Kisspeptin signaling is required for peripheral but not central stimulation of gonadotropin-releasing hormone neurons by NMDA. J. Neurosci. 30, 8581–8590 (2010).
Urbanski, H.F. & Ojeda, S.R. A role for N-methyl-D-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology 126, 1774–1776 (1990).
Wierman, M.E., Kiseljak-Vassiliades, K. & Tobet, S. Gonadotropin-releasing hormone (GnRH) neuron migration: initiation, maintenance and cessation as critical steps to ensure normal reproductive function. Front. Neuroendocrinol. 32, 43–52 (2011).
Forni, P.E. & Wray, S. GnRH, anosmia and hypogonadotropic hypogonadism--where are we? Front. Neuroendocrinol. 36, 165–177 (2015).
Prevot, V. et al. Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J. Neurosci. 23, 230–239 (2003).
Prevot, V. Puberty in mice and rats. in Knobil and Neill′s Physiology of Reproduction (eds. Plant, T.M. & Zeleznik, J.) 1395–1439 (Elsevier, New York, 2015).
Kuiri-Hänninen, T., Sankilampi, U. & Dunkel, L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm. Res. Paediatr. 82, 73–80 (2014).
Belsham, D.D. & Mellon, P.L. Transcription factors Oct-1 and C/EBPbeta (CCAAT/enhancer-binding protein-beta) are involved in the glutamate/nitric oxide/cyclic-guanosine 5′-monophosphate-mediated repression of mediated repression of gonadotropin-releasing hormone gene expression. Mol. Endocrinol. 14, 212–228 (2000).
Lee, V.H., Lee, L.T. & Chow, B.K. Gonadotropin-releasing hormone: regulation of the GnRH gene. FEBS J. 275, 5458–5478 (2008).
Burk, U. et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 9, 582–589 (2008).
Le Béchec, A. et al. MIR@NT@N: a framework integrating transcription factors, microRNAs and their targets to identify sub-network motifs in a meta-regulation network model. BMC Bioinformatics 12, 67 (2011).
van Rooij, E., Purcell, A.L. & Levin, A.A. Developing microRNA therapeutics. Circ. Res. 110, 496–507 (2012).
Alisi, A. et al. Mirnome analysis reveals novel molecular determinants in the pathogenesis of diet-induced nonalcoholic fatty liver disease. Lab. Invest. 91, 283–293 (2011).
Klein, D. et al. MicroRNA expression in alpha and beta cells of human pancreatic islets. PLoS One 8, e55064 (2013).
Dweep, H., Sticht, C., Pandey, P. & Gretz, N. miRWalk--database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J. Biomed. Inform. 44, 839–847 (2011).
Costinean, S. et al. Src homology 2 domain-containing inositol-5-phosphatase and CCAAT enhancer-binding protein beta are targeted by miR-155 in B cells of Emicro-MiR-155 transgenic mice. Blood 114, 1374–1382 (2009).
Koch, M., Mollenkopf, H.J., Klemm, U. & Meyer, T.F. Induction of microRNA-155 is TLR- and type IV secretion system-dependent in macrophages and inhibits DNA-damage induced apoptosis. Proc. Natl. Acad. Sci. USA 109, E1153–E1162 (2012).
Rodriguez, A. et al. Requirement of bic/microRNA-155 for normal immune function. Science 316, 608–611 (2007).
Yang, J.H., Li, J.H., Jiang, S., Zhou, H. & Qu, L.H. ChIPBase: a database for decoding the transcriptional regulation of long non-coding RNA and microRNA genes from ChIP-Seq data. Nucleic Acids Res. 41, D177–D187 (2013).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Lefterova, M.I. et al. Cell-specific determinants of peroxisome proliferator-activated receptor gamma function in adipocytes and macrophages. Mol. Cell. Biol. 30, 2078–2089 (2010).
Novaira, H.J., Fadoju, D., Diaczok, D. & Radovick, S. Genetic mechanisms mediating kisspeptin regulation of GnRH gene expression. J. Neurosci. 32, 17391–17400 (2012).
Hanchate, N.K. et al. Kisspeptin-GPR54 signaling in mouse NO-synthesizing neurons participates in the hypothalamic control of ovulation. J. Neurosci. 32, 932–945 (2012).
Bellefontaine, N. et al. Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction. J. Clin. Invest. 124, 2550–2559 (2014).
Rameau, G.A. et al. Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the NMDA receptor regulates AMPA receptor trafficking and neuronal cell death. J. Neurosci. 27, 3445–3455 (2007).
Bouret, S.G., Draper, S.J. & Simerly, R.B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 24, 2797–2805 (2004).
Caron, E., Ciofi, P., Prevot, V. & Bouret, S.G. Alteration in neonatal nutrition causes perturbations in hypothalamic neural circuits controlling reproductive function. J. Neurosci. 32, 11486–11494 (2012).
Grueter, C.E. et al. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell 149, 671–683 (2012).
Lomniczi, A. et al. Epigenetic control of female puberty. Nat. Neurosci. 16, 281–289 (2013).
Lomniczi, A. et al. Epigenetic regulation of puberty via Zinc finger protein-mediated transcriptional repression. Nat. Commun. 6 http://dx.doi.org/10.1038/ncomms10195 (2015).
Issler, O. et al. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron 83, 344–360 (2014).
Hanchate, N.K. et al. SEMA3A, a gene involved in axonal pathfinding, is mutated in patients with Kallmann syndrome. PLoS Genet. 8, e1002896 (2012).
Franzoni, E. et al. miR-128 regulates neuronal migration, outgrowth and intrinsic excitability via the intellectual disability gene Phf6. eLife 4 http://dx.doi.org/10.7554/eLife.04263 (2015).
Givens, M.L. et al. Developmental regulation of gonadotropin-releasing hormone gene expression by the MSX and DLX homeodomain protein families. J. Biol. Chem. 280, 19156–19165 (2005).
Cottrell, E.C., Campbell, R.E., Han, S.K. & Herbison, A.E. Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology 147, 3652–3661 (2006).
Garthwaite, J. From synaptically localized to volume transmission by nitric oxide. J. Physiol. (Lond.) 594, 9–18 (2016).
Cossenza, M. et al. Nitric oxide in the nervous system: biochemical, developmental, and neurobiological aspects. Vitam. Horm. 96, 79–125 (2014).
Choe, H.K. et al. Real-time GnRH gene transcription in GnRH promoter-driven luciferase-expressing transgenic mice: effect of kisspeptin. Neuroendocrinology 102, 194–199 (2015).
Parent, A.S. et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr. Rev. 24, 668–693 (2003).
Chachlaki, K. & Prévot, V. Coexpression profiles reveal hidden gene networks. Proc. Natl. Acad. Sci. USA 113, 2563–2565 (2016).
Boehm, U. et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism—pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 11, 547–564 (2015).
Spergel, D.J., Krüth, U., Hanley, D.F., Sprengel, R. & Seeburg, P.H. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J. Neurosci. 19, 2037–2050 (1999).
García-Galiano, D. et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology 153, 316–328 (2012).
Messina, A. et al. Dysregulation of Semaphorin7A/β1-integrin signaling leads to defective GnRH-1 cell migration, abnormal gonadal development and altered fertility. Hum. Mol. Genet. 20, 4759–4774 (2011).
Giacobini, P. et al. Brain endothelial cells control fertility through ovarian-steroid-dependent release of semaphorin 3A. PLoS Biol. 12, e1001808 (2014).
Beauvillain, J.C. & Tramu, G. Immunocytochemical demonstration of LH-RH, somatostatin, and ACTH-like peptide in osmium-postfixed, resin-embedded median eminence. J.Histochem.Cytochem. 28, 1014–1017 (1980).
Schmittgen, T.D. & Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008).
Acknowledgements
A.M. was a postdoctoral fellow supported by the Fondation pour la Recherche Médicale (FRM). We are indebted to D. Accili for his help in conducting the ChIP assays. We thank M. Tardivel (microscopy core facility), M.-H. Gevaert (histology core facility), D. Taillieu and J. Devassine (animal core facility) and the BICeL core facility of the Lille University School of Medicine for expert technical assistance. This research was supported by the FRM (Equipe FRM 2005 and DEQ20130326524, France to V.P.), grant BFI2011-025021 from the Spanish Ministry of Economy and Science (M.T.-S.), the ERC COST action BM1015 (V.P., P.G. and M.T.-S.) and the Fondation Bettencourt Schueller (F.L.).
Author information
Authors and Affiliations
Contributions
A.M., F.L., K.C., J.R., S.R., F.G., M.T.-S., P.G. and V.P. designed the experiments. A.M., F.L., K.C., J.R., N.J., S.G., F.G., J.P., P.G. and V.P. performed the experiments. A.M. and V.P. analyzed the data. All authors discussed the results and A.M., J.R., S.R., M.T.-S., P.G. and V.P. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 MicroRNA biogenesis and post-transcriptional gene silencing.
MicroRNAs are produced in the nucleus by the transcription of long primary microRNAs (pri-miRNA) from miRNA genes. After cleavage by the Drosha nuclear RNase III, the precursor miRNA (pre-miRNA) is exported to the cytoplasm by exportin via a nuclear pore. In the cytoplasm, the pre-miRNA is processed by the RNase activity of Dicer to a mature microRNA duplex that is in turn incorporated into the RNA Induced Silencing Complex (RISC). Usually, only one of the mature miRNA strands is used by RISC as a scaffold to bind in a sequence-specific manner to the 3’UTR of target mRNAs, inducing their posttranscriptional repression by degradation or by interfering with translation.
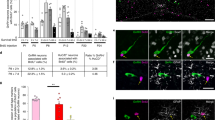
Supplementary Figure 2 Selective invalidation of Dicer in GnRH neurons causes central hypogonadotropic hypogonadism in mice.
(a and b) Western blot showing Tomato expression in the hypothalamus but not the gonads of adult GnRH::cre; dtTomatoloxP/STOP mice in male (a) and female (b) mice. Actin was used as a loading control. Ctx, cerebral cortex; POA, preoptic region of the hypothalamus; O, ovary; T, testis. Full-length blots are presented in Supplementary Figure 15. (c) Adult Gnrh::Cre; DicerloxP/loxP mice exhibit low uterine weights when compared to their wild-type DicerloxP/loxP littermates even though the uterus remains responsive to gonadal steroid treatment (E: estradiol, P: progesterone) after ovariectomy (OVX) (two-way ANOVA; treatment, F(1,14)=69.41, p<0.0001; genotype, F(1,14)=128.6, p<0.0001; interaction, F(1,14)=13.76, p<0.0001, n= 3,4,5 and 6 mice per group; Ctrl DicerloxP/loxP vs. Ctrl Gnrh::Cre; DicerloxP/loxP, q(14)=6.89, p=0.0013; Ctrl DicerloxP/loxP vs. OVX E+P DicerloxP/loxP, q(14)=12.37, p=0.0001; Ctrl DicerloxP/loxP vs. OVX E+P Gnrh::Cre; DicerloxP/loxP, q(14)=3.21, p=0.152; Ctrl Gnrh::Cre; DicerloxP/loxP vs. OVX E+P DicerloxP/loxP, q(14)=18.56, p<0.0001; Gnrh::Cre; DicerloxP/loxP vs. OVX E+P Gnrh::Cre; DicerloxP/loxP, q(14)=4.505, p=0.0.0298; OVX E+P DicerloxP/loxP vs. OVX E+P Gnrh::Cre; DicerloxP/loxP, q(14)=17.13, p<0.0001). Adult OVX mice received a single subcutaneous injection of 17β-estradiol 3-benzoate (E, 1 μg/20 g of body weight in sesame oil) at 9:00 a.m. The following day, animals received another injection of progesterone (P, 500 μg/20 g body weight, s.c., in sesame oil) at 9:00 a.m. In cycling DicerloxP/loxP mice, uteri were collected on the day of proestrus (Ctrl). (*: p<0.05; **: p<0.01; ***: p< 0.001) (d) Male Gnrh::Cre; DicerloxP/loxP mice exhibit hypogonadism (testicular weight: t-test, t(15)=16.2, p<0.0001, n=6 and 11 animals per group). (f to g) While intracerebroventricular infusion of the glutamate receptor agonist NMDA (1 nM, 15 min) (F) or of kisspeptin (Kiss-10, 1 nM, 15 min) (g) fails to promote LH release in male mutant mice (NMDA, paired t-test, t(5)=1.54, p=0.183, n=6; Kp-10, paired t-test, t(5)=0.09, p=0.93, n=6 mice) when compared to control male mice (NMDA, paired t-test, t(10)=5.44, p=0.0003, n=11 mice; Kp-10, paired t-test, t(9)=5.85, p=0.0002, n=10 mice), the intraperitoneal injection of GnRH (0.25 μg, 15 min) incites LH release in both mutant and control male littermates (DicerloxP/loxP, paired t-test, t(9)=7, p=0.0001, n=10; Gnrh::Cre; DicerloxP/loxP, paired t-test, t(4)=9.8, p=0.0006, n=5 mice) (e). Values shown are mean ± SEM. (h) Gnrh::Cre; DicerloxP/loxP mice respond to gonadotropin priming by ovulation. Ten-week-old females were injected i.p. with 5 U pregnant mare serum gonadotropins (PMSG,,Sigma-Aldrich Co.) between 1:00 and 4:00 p.m. of day 1. On the morning of day 3 (between 10:00 and 11:00 a.m.), mice received an injection of 5 U hMG-Lepori (Angelini Pharma Inc.) containing 5 U hCG/5 U FSH. Two days later, the animals were sacrificed and ovaries were collected and immersed in 4% PFA until histological analysis. Successful ovulation has been obtained in 5 distinct mutant mice.
Supplementary Figure 3 GnRH peptide immunoreactivity is lost in adult mice in which GnRH neurons are deficient for Dicer expression.
Representative photomicrographs of GnRH immunofluorescence (IR) in sagittal brain sections from adult mice. AC, anterior commissure; CC, corpus callosum; CX, cerebral cortex; OB, olfactory bulb; OC, optic chiasm; POA, preoptic area; SEP, medial septum. Arrows in the top panels show the areas from the OB and SEP that are magnified in the bottom panels. These images are representative of what has been observed in more than 4 adult animals per group.
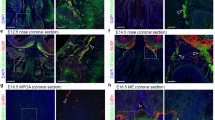
Supplementary Figure 4 Selective invalidation of Dicer expression in GnRH neurons does not cause migratory defects during embryogenesis.
(a) Schematic diagram illustrating the GnRH neuronal migratory pathway from the nose to the brain (arrow) during embryogenesis in a sagittal view of a mouse embryo head. nfj, nasal forebrain junction; bfb, basal forebrain; vno vomeronasal organ; LV, lateral ventricle; 3rdV, third ventricle; 4thV, fourth ventricle. (b) Distribution analysis of GnRH-immunoreactive (IR) neurons along the migratory route in mutant (red bars) and control (blue bars) mice at embryonic day 14.5 (E14.5) and at birth (P0). E14.5, n=3 animals per group: nose, t(4)=0.19, p=0.86; nfj, t(4)=0.47, p=0.66; bfb, t(4)=0.45, p=0.67; total, t(4)=0.48, p=0.65. P0, t(4)=0.27, p=0.80, n=3 mice per group. (c) Representative photomicrographs showing GnRH neuronal cell body and axon terminal distribution in the preoptic region and the median eminence, respectively, of mutant and control mice at P0 in coronal sections. OVLT, organum vasculosum of the lamina terminalis. These images are representative of what has been observed in the 3 animals studied at P0 per group. Scale bar, 200 μm. Values shown are means ± SEM.
Supplementary Figure 5 Selective invalidation of Dicer expression in GnRH neurons leads to the progressive loss of GnRH peptide expression during postnatal development.
Representative photomicrographs of GnRH immunoreactivity taken from Gnrh::Cre; DicerloxP/loxP and DicerloxP/loxP littermates at different ages. OVLT, organum vasculosum of the lamina terminalis. These images are representative of what has been observed in the 3, 5, 5, 10 and 4 animals studied at P0, P12, P21, P28 and P60, respectively, per group. Scale bar, 200 μm.
Supplementary Figure 6 GnRH neurons deficient for Dicer expression lose Gnrh promoter activity during postnatal development.
Quantitative comparison of the number of GnRH neurons expressing the green fluorescent protein (GFP) in Dicer mutant (red bars) and control (green bars) mice at different stages of postnatal development. Two-way ANOVA, time: F(2,17)=18.24, p<0.0001; genotype: F(1,17)=109.2, p<0.0001; interaction: F(2,17)=3.571, p=0.0507; Sidak’s multiple comparison test: P12, t(17)=3.99, p=0.0028, n=3-4 per group; P21, t(17)=5.727, p<0.0001, n=3 per group; P28, t-test, t(17)=9.012, p<0.0001, n=8 per group (**: p<0.01, ***: p<0.001). Values shown are means ± SEM.
Supplementary Figure 7 Isolation of hypothalamic GnRH neurons in postnatal mice.
GnRH-GFP neuron isolation by FACS and real-time PCR analysis of GnRH mRNA expression in GFP-positive (POS) and negative (NEG) cells (t-test, t(3)=7.79, p=0.016, n=3 per group). Values shown are means ± SEM.
Supplementary Figure 8 Zeb1 is a putative repressor of Gnrh promoter activator genes and binds to the Gnrh promoter itself.
(a) The miRNAs whose expression is specifically enriched in GnRH neurons at P12 (Fig. 3D-F) include members of the miR-200 family, which is known to control Zeb1 expression. (b) Distribution of putative Zeb1-binding sites in the upstream region of GnRH modulator genes as well as in GnRH and Gpr54 genes. (c) Diagram showing the distribution of putative Zeb1 binding sites in the human GNRH gene (upper panel) and their validation using a Zeb1 chromatin immunoprecipitation assay in an immortalized mouse cell line secreting GnRH and cultured in the presence or absence of fetal bovine serum (fbs). Values are expressed relative to the immunoprecipitation of chromatin containing the GnRH promoter region with irrelevant IgG species, arbitrarily set at 1 (dotted red line). T-test, serum-free medium (sfm) vs. fbs: Enh1, t(6)=19.42, p<0.0001; Enh2, t(6)=2.98, p=0.0245; Enh-Pro1, t(6)=0.05, p=0.9648; Pro1, t(6)=2.88, p=0.0279; Pro2, t(6)=2.08, p=0.0823, n=4 per group. (*: p<0.05, ***: p<0.001). (d) A target site blocker (TSB) designed to selectively impair the ability of miR-200b/200c/429 to target the Zeb1 transcript in the infantile hypothalamus. (e) Putative double negative-feedback loop illustrating the potential contribution of mir200, Zeb1 and Pouf2f1, which are both known to control the expression of the former in the infantile control of GnRH gene expression. Values shown are means ± SEM.
Supplementary Figure 9 Use of target site blockers to selectively impair the ability of miR-155 to target the Cebpb transcript in the infantile hypothalamus.
(a) When infused into the lateral ventricle of infantile mice, TSBs (red fluorescence) reach the hypothalamus and are taken up by GnRH neurons (green fluorescence). Cell nuclei are visualized in blue using Hoechst. Scale bars, 100 μm (15 μm in inset). These images are representative of what has been observed in more than 3 animals. (b) Sequences corresponding to the immature and mature forms of miR-155. (c) Schematic diagram illustrating the predicted consequential pairing of miR-155 (top) with the target region (bottom). (d and e) Strategy used to selectively block the binding of miR-155, which has many gene targets, to Cebpb (d). The TSB sequence encompasses the miR-155 binding site and contains an additional motif that selectively hybridizes with Cebpb (e). (f) Effects of L-NAME treatment on the expression of GnRH promoter activators potentially regulated by Zeb-1 in infantile Gnrh::Cre; DicerloxP/loxP mice at P12 (t-test: Aes, t(7)=1.76, p=0. 121; Dlx1, t(8)=1.94, p=0.089; Meis1, t(9)=0.97, p=0.358; Otx2, t(9)=0.098, p=0.924; Pbx1, t(9)=1.53, p=0.159; Pxnox1, t(10)=0.633, p=0.541, n=5-6 per group). Values shown are means ± SEM. (g) Schematic representation of a miRNA-gene network potentially regulating GnRH expression in the infantile period, and its crosstalk with nitrergic neurotransmission.
Supplementary Figure 10 MicroRNA-155 enrichment in GnRH neurons and effects of TSBs on Cebpb and Zeb1 transcripts expression in non-GnRH cells.
RT-PCR analysis of the expression of miR-155 in FACS-sorted cells from preoptic explants isolated from Gnrh::Gfp mice wild-type for Dicer (a; t-test Pos vs. Neg: t(4)=4.679, p=0.0095; n=3-4 per group) and of the expression of Cebpb (b; one-way ANOVA: F(2, 15)=4.302, p=0.0334, n=6 per group; TSB-155, p=0.0275; TSB-200, p>0.9999) and Zeb1 (c; one-way ANOVA: F(2, 15)=4.302, p=0.7476, n=6 per group; TSB-155, p=0.3764; TSB-200, p=0.9905) in GnRH-negative cells at P12. Values shown are means ± SEM.
Supplementary Figure 11 Distribution of C/EBPβ-binding sites in the Zeb1 and Gpr54 (KISSR1) genes.
(a,b) In silico analyses of C/EBPβ-binding sites in the Zeb1 (a) and Gpr54 (KISSR1) (b) genes. (c) Putative miRNA-gene network illustrating the convergent contributions of miR200 and miR155, and their respective target genes Zeb1 and Cebpb, in the infantile control of GnRH expression via the repression of the kisspeptin/Gpr54/Otx2-signaling pathway.
Supplementary Figure 12 Effect of TSB treatment on growth and puberty onset.
(a) Growth curve of female wild-type mice that received a single bolus injection of the same TSB-200 and/or TSB-155 into the brains at P9. Two-way repeated measures ANOVA, time: F(6,192)=1010, p<0.0001; treatment: F(3,32)=2.109, p=0.119; interaction: F(18,192)=1.570, p=0.071; subject matching: F(32,192)=12.88, p<0.0001, n= 8-10 per group. Values shown are means ± SEM. (b) Schematic diagrams illustrating the rebound hypothesis.
Supplementary Figure 13 Neuronal microcircuit and miRNA-gene network in hypothalamic GnRH neurons regulating the onset of puberty.
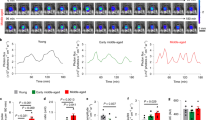
Schematic diagrams illustrating the potential contribution of the miR-155 and miR-200 families and their target genes to the increase in GnRH gene expression during the infantile period of postnatal development, and how these events could be intertwined with the integration of postmigratory GnRH neurons into the neural network responsible for regulating the timely onset of puberty. GnRH neuroglial network maturation (top panels). Although the morphological development of the hypothalamus is almost complete at birth, axons of the neurons located in the arcuate nucleus of the hypothalamus (ARH), which are thought to mediate at least part of the effects of gonadal steroid on the HPG axis, first reach the preoptic region during the infantile period, when astrogliogenesis, synaptogenesis, and dendritic pruning are also thought to occur. These maturational events are well positioned to trigger the miRNA-evoked changes in the transcription factor-gene network controlling GnRH promoter activity during the infantile period, which we have uncovered here. Hormonal profiles (bottom panel). The hormonal profiles illustrated in the schematic are those for a female mouse. In females, the first postnatal activational period of the HPG axis coincides with the arrival of ARH fibers in the preoptic region, which results in an infantile surge of FSH that triggers the growth of the first pool of ovarian follicles, destined to ovulate at puberty, as well as the sporadic elevation of LH levels that contributes to their maturation. This critical time-window for sexual maturation is highly reminiscent of "mini-puberty" in humans16. The second activational period occurs during the peripubertal period, when a diurnal rhythm of LH release that accelerates the functional development of the ovaries is established. A third and final activational period coincides with the moment when ovarian follicles reach full maturity, i.e. the Graafian stage, and release massive amounts of ovarian steroids, specifically estrogens, which exert a positive-feedback effect on the HPG, coordinating the onset of the first preovulatory GnRH and LH/FSH surge and triggering the first ovulation, thus conferring fertility on the individual. In males, as in females, the primary events that initiate the onset of puberty originate within the hypothalamus (see for review15).
Supplementary Figure 14 Expression of Gnrh1, Zeb1 and Cebpb transcripts in the human brain diencephalon.
The expression of these genes is indicated by dots and is mapped onto a sagittal view of a reference brain. The color of the dots indicates the expression level: green (low expression) through red (high expression. Reported values are the average expression energy per region from 6 probands normalized to the average expression across the whole brain and then log2-transformed. Images were produced by Brain explorer 2 software, an interactive version of the Allen Human Brain Atlas, using the in situ hybridization probes GNRH1- A_23_P254594, ZEB1-CUST_13871_PI416261804 and CEBPB- A_23_P411296.
Supplementary Figure 15 Full-length blots
(a) Full-length blots illustrated in Fig. 5e. (b) Full-length blots illustrated in Supplementary Figure 2a. Note that only the first 6 lanes (T, T, Ctx, Ctx, POA, POA) were used for illustration. (c) Full-length blots illustrated in Supplementary Figure 2b. Note that only the first 5 lanes (O, O, Ctx, POA, POA) were used for illustration.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 and Supplementary Tables 1–5 (PDF 4808 kb)
Rights and permissions
About this article
Cite this article
Messina, A., Langlet, F., Chachlaki, K. et al. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nat Neurosci 19, 835–844 (2016). https://doi.org/10.1038/nn.4298
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4298
This article is cited by
-
Metabolic control of puberty: 60 years in the footsteps of Kennedy and Mitra’s seminal work
Nature Reviews Endocrinology (2024)
-
Dicer ablation in Kiss1 neurons impairs puberty and fertility preferentially in female mice
Nature Communications (2022)
-
Ontogenetic rules for the molecular diversification of hypothalamic neurons
Nature Reviews Neuroscience (2022)
-
Efficacy of short-term induction therapy with low-dose testosterone as a diagnostic tool in the workup of delayed growth and puberty in boys
Journal of Endocrinological Investigation (2022)
-
Prolonged breastfeeding protects from obesity by hypothalamic action of hepatic FGF21
Nature Metabolism (2022)