Abstract
The ventral tegmental area (VTA) is best known for its dopamine neurons, some of which project to nucleus accumbens (nAcc). However, the VTA also has glutamatergic neurons that project to nAcc. The function of the mesoaccumbens glutamatergic pathway remains unknown. Here we report that nAcc photoactivation of mesoaccumbens glutamatergic fibers promotes aversion. Although we found that these mesoaccumbens glutamatergic fibers lack GABA, the aversion evoked by their photoactivation depended on glutamate- and GABA-receptor signaling, and not on dopamine-receptor signaling. We found that mesoaccumbens glutamatergic fibers established multiple asymmetric synapses on single parvalbumin GABAergic interneurons and that nAcc photoactivation of these fibers drove AMPA-mediated cellular firing of parvalbumin GABAergic interneurons. These parvalbumin GABAergic interneurons in turn inhibited nAcc medium spiny output neurons, thereby controlling inhibitory neurotransmission in nAcc. To our knowledge, the mesoaccumbens glutamatergic pathway is the first glutamatergic input to nAcc shown to mediate aversion instead of reward, and the first pathway shown to establish excitatory synapses on nAcc parvalbumin GABAergic interneurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Carlezon, W.A. Jr. & Thomas, M.J. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 56, 122–132 (2009).
Todtenkopf, M.S. et al. Brain reward regulated by AMPA receptor subunits in nucleus accumbens shell. J. Neurosci. 26, 11665–11669 (2006).
Britt, J.P. et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76, 790–803 (2012).
Friedman, D.P., Aggleton, J.P. & Saunders, R.C. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the Macaque brain. J. Comp. Neurol. 450, 345–365 (2002).
Grace, A.A., Floresco, S.B., Goto, Y. & Lodge, D.J. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 30, 220–227 (2007).
Kelley, A.E. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44, 161–179 (2004).
O'Donnell, P. & Grace, A.A. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J. Neurosci. 15, 3622–3639 (1995).
Phillips, P.E., Stuber, G.D., Heien, M.L., Wightman, R.M. & Carelli, R.M. Subsecond dopamine release promotes cocaine seeking. Nature 422, 614–618 (2003).
Stuber, G.D. et al. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science 321, 1690–1692 (2008).
Sesack, S.R., Carr, D.B., Omelchenko, N. & Pinto, A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann. NY Acad. Sci. 1003, 36–52 (2003).
Papp, E. et al. Glutamatergic input from specific sources influences the nucleus accumbens-ventral pallidum information flow. Brain Struct. Funct. 217, 37–48 (2012).
Stuber, G.D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011).
Morales, M. & Root, D.H. Glutamate neurons within the midbrain dopamine regions. Neuroscience 282, 60–68 (2014).10.1016/j.neuroscience.2014.05.032
Stuber, G.D., Hnasko, T.S., Britt, J.P., Edwards, R.H. & Bonci, A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J. Neurosci. 30, 8229–8233 (2010).
Tecuapetla, F. et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J. Neurosci. 30, 7105–7110 (2010).
Van Bockstaele, E.J. & Pickel, V.M. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 682, 215–221 (1995).
van Zessen, R., Phillips, J.L., Budygin, E.A. & Stuber, G.D. Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194 (2012).
Yamaguchi, T., Wang, H.L., Li, X., Ng, T.H. & Morales, M. Mesocorticolimbic glutamatergic pathway. J. Neurosci. 31, 8476–8490 (2011).
Oleson, E.B., Gentry, R.N., Chioma, V.C. & Cheer, J.F. Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J. Neurosci. 32, 14804–14808 (2012).
Peciña, S. & Berridge, K.C. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered 'wanting' for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur. J. Neurosci. 37, 1529–1540 (2013).
Brown, M.T. et al. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature 492, 452–456 (2012).
Kawano, M. et al. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J. Comp. Neurol. 498, 581–592 (2006).
Trudeau, L.E. et al. The multilingual nature of dopamine neurons. Prog. Brain Res. 211, 141–164 (2014).
Yamaguchi, T., Sheen, W. & Morales, M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur. J. Neurosci. 25, 106–118 (2007).
Chuhma, N., Mingote, S., Moore, H. & Rayport, S. Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron 81, 901–912 (2014).
Zhang, S. et al. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat. Neurosci. 18, 386–392 (2015).
Root, D.H., Mejias-Aponte, C.A., Qi, J. & Morales, M. Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. J. Neurosci. 34, 13906–13910 (2014).
Yamaguchi, T., Qi, J., Wang, H.L., Zhang, S. & Morales, M. Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur. J. Neurosci. 41, 760–772 (2015).
Root, D.H. et al. Single rodent mesohabenular axons release glutamate and GABA. Nat. Neurosci. 17, 1543–1551 (2014).
Tritsch, N.X., Ding, J.B. & Sabatini, B.L. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 490, 262–266 (2012).
Taylor, S.R. et al. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J. Comp. Neurol. 522, 3308–3334 (2014).
Kawaguchi, Y., Wilson, C.J., Augood, S.J. & Emson, P.C. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 18, 527–535 (1995).
Kita, H., Kosaka, T. & Heizmann, C.W. Parvalbumin-immunoreactive neurons in the rat neostriatum: a light and electron microscopic study. Brain Res. 536, 1–15 (1990).
Li, X., Qi, J., Yamaguchi, T., Wang, H.L. & Morales, M. Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Struct. Funct. 218, 1159–1176 (2013).
Parthasarathy, H.B. & Graybiel, A.M. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J. Neurosci. 17, 2477–2491 (1997).
Ramanathan, S., Hanley, J.J., Deniau, J.M. & Bolam, J.P. Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J. Neurosci. 22, 8158–8169 (2002).
Tepper, J.M., Wilson, C.J. & Koós, T. Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Res. Rev. 58, 272–281 (2008).
Koós, T. & Tepper, J.M. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat. Neurosci. 2, 467–472 (1999).
Gittis, A.H., Nelson, A.B., Thwin, M.T., Palop, J.J. & Kreitzer, A.C. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J. Neurosci. 30, 2223–2234 (2010).
Planert, H., Szydlowski, S.N., Hjorth, J.J., Grillner, S. & Silberberg, G. Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways. J. Neurosci. 30, 3499–3507 (2010).
Taverna, S., Canciani, B. & Pennartz, C.M. Membrane properties and synaptic connectivity of fast-spiking interneurons in rat ventral striatum. Brain Res. 1152, 49–56 (2007).
Koos, T., Tecuapetla, F. & Tepper, J.M. Glutamatergic signaling by midbrain dopaminergic neurons: recent insights from optogenetic, molecular and behavioral studies. Curr. Opin. Neurobiol. 21, 393–401 (2011).
Cachope, R. et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2, 33–41 (2012).
Threlfell, S. et al. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75, 58–64 (2012).
Chang, H.T. & Kita, H. Interneurons in the rat striatum: relationships between parvalbumin neurons and cholinergic neurons. Brain Res. 574, 307–311 (1992).
Barker, D.J., Root, D.H., Zhang, S. & Morales, M. Multiplexed neurochemical signaling by neurons of the ventral tegmental area. J. Chem. Neuroanat. http://dx.doi.org/10.1016/j.jchemneu.2015.12.016 (4 January 2016).10.1016/j.jchemneu.2015.12.016
Wang, H.L., Qi, J., Zhang, S., Wang, H. & Morales, M. Rewarding effects of optical stimulation of ventral tegmental area glutamatergic neurons. J. Neurosci. 35, 15948–15954 (2015).
Borgius, L., Restrepo, C.E., Leao, R.N., Saleh, N. & Kiehn, O. A transgenic mouse line for molecular genetic analysis of excitatory glutamatergic neurons. Mol. Cell. Neurosci. 45, 245–257 (2010).
Qi, J. et al. A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons. Nat. Commun. 5, 5390 (2014).
Li, B. et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 470, 535–539 (2011).
Acknowledgements
We thank O. Kiehn and L. Borgius (Karolinska Institutet, Solna, Sweden) for providing the VGluT2::Cre transgenic mice. We thank R. Wise, M.F. Barbano and D. Root for comments. We thank B. Liu and R. Ye for their help in processing brain tissue. This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, US National Institutes of Health (IRP/NIDA/NIH).
Author information
Authors and Affiliations
Contributions
M.M. conceptualized the project. J.Q. performed behavioral and pharmacological studies. J.Q. and D.J.B. analyzed data from behavioral experiments. J.Q., S.Z. and H.-L.W. performed neuroanatomy and immunolabeling studies. H.-L.W. performed in situ hybridization. J.Q. and S.Z. performed confocal microscopy studies. S.Z. performed electron microscopy studies. J.M.-B. performed electrophysiological studies. All authors participated in conception, experimental design and data interpretation. M.M. wrote the manuscript with the participation of all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Histological verification of viral infection in the VTA of VGluT2::Cre mice.
(a-b)Virus (AAV-DIO-ChR2-eYFP) infected VGluT2 neurons are restricted to the VTA. Double labeled coronal sections for detection of eYFP (green) and TH (red) from rostral (−2.8 mm) to caudal (−3.9 mm) levels of the midbrain. SNC: substantia nigra compacta.
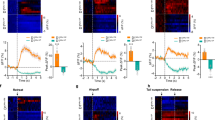
Supplementary Figure 2 Photostimulation of nAcc mShell fibers from VTA VGluT2 neurons produces place aversion in mice.
(a) Histological verification of optical fiber placements within the nAcc-mShell. Representative image showing VTA-VGluT2-inputs (eYFP-green) in the nAcc-mShell and diagrams corresponding to optical fiber placements in the nAcc-mShell of VGluT2::Cre mice. Sections were counterstained with the nuclear dye DAPI (blue). Optical fibers placements are indicated by blue lines (VGluT2-ChR2-eYFP group) or pink lines (VGluT2-eYFP group). aca, anterior commissure anterior part; CPu, caudate putamen; Core, nucleus accumbens core; mShell, nucleus accumbens medial shell. (b) VGluT2-ChR2-eYFP mice (n = 6) spent significantly more time in the photostimulation-paired chamber than the unpaired chamber at a stimulation frequency of 10 Hz or 20 Hz, but not at 5 Hz or 40 Hz (F6,40 = 0.64, P = 0.70; two-way ANOVA, * P < 0.05). (c) Tracking data show representative traces from a VGluT2-ChR2-eYFP mouse (left panel) and a VGluT2-eYFP mouse (right panel) on test day. On test day when photostimulation was no longer available, VGluT2-ChR2-eYFP mice avoided the chamber previously-paired with photostimulation. (d) Photostimulation does not affect travel distance by VGluT2-ChR2-eYFP mice in the conditioning chambers on conditioning day (main effect group: F1,18 = 0.202, P = 0.659; two-way ANOVA). (e) VGluT2-ChR2-eYFP mice avoid the photostimulation-paired chamber on both the photostimulation conditioning days and on the first test day (as shown in Fig. 2, * P < 0.05, Newman-Keuls post hoc test), but on a second test day (24 h after the first test day) in which mice spent similar time in the photostimulation-paired and photostimulation-unpaired chambers (n.s. = no significant difference, P = 0.997). Data are represented as mean + SEM.
Supplementary Figure 3 Histological verification of cannula placement for pharmacological studies of real-time place aversion induced by photostimulation of nAcc mShell fibers from VTA VGluT2 neurons.
(a) The microinjection needle protruded 1 mm to the microinjection cannula tips in nAcc. The cannula tips are indicated by green triangles (ACSF group, n = 11), brown squares (MK801 + CNQX group, n = 6), pink circles (bicuculline + saclofen group, n = 7), blue circles (SCH23390 group, n = 7), orange squares (eticlopride group, n = 7) or red triangles (SCH23390 + eticlopride group, n = 6). aca, anterior commissure anterior part; CPu, caudate putamen; Core, accumbens nucleus core; mShell, accumbens nucleus medial shell. (b) Travel distance during the conditioning day. The average travel distances for each group of mice are represented as mean + SEM. MK801 + CNQX significantly increased the locomotor activity of VGluT2-ChR2-eYFP mice on training day (F5,38 = 5.49, P = 0.0007, one-way ANOVA; * P = 0.021).
Supplementary Figure 4 FluoroGold (FG) injection sites in nAcc mShell and frequency of seven different phenotypes of VTA neurons projecting to nAcc mShell.
(a) Schematic representation of FG injection sites within the mouse nAcc-mShell (3 mice). Diagrams corresponding to the injection sites arranged rostrocaudally from +1.70 mm to +0.86 mm. (b) Frequency of seven different phenotypes of VTA neurons innervating the nAcc-mShell (mean + SEM). Among the 675 identified VTA neurons projecting to the nAcc-mShell, 69.3% expressed only TH, 18.7% coexpressed VGluT2 mRNA and TH, 7.7% expressed only VGluT2 mRNA, 2.3% coexpressed VGluT2 mRNA and GADs mRNA, 0.8% coexpressed TH and GADs mRNA, 0.5% expressed only GADs mRNA, and 0.7% coexpressed VGluT2 mRNA, TH and GADs mRNA. FG neurons were counted between bregma −3.08 mm and −3.88 mm (n = 3 mice, 12–13 sections per mouse).
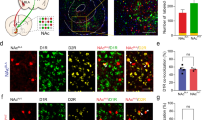
Supplementary Figure 5 Injection of 6-OHDA resulted in the loss of TH in the nAcc mShell without eliminating aversion induced by photoactivation of mesoaccumbens glutamatergic fibers, and without eliminating synapses between PV dendrites and terminals from VTA VGluT2 neurons.
(a) TH terminals lesion was induced by unilateral injection of 6-OHDA into the nAcc-mShell. aca, anterior commissure anterior part; Core, nucleus accumbens core; CPu, caudate putamen; LV, lateral ventricle; mShell, nucleus accumbens medial shell. (b) High magnification image shows the loss of TH in the nAcc-mShell after 6-OHDA injection, and the contralateral mShell maintained the normal level of TH after saline injection. (c) nAcc-mShell photostimulation of mesoaccumbens-fibers promoted real-time place aversion, which remained after 6-OHDA lesions (n = 9, 6-OHDA group; n = 13, vehicle group; main effect group: F1,20 = 0.03, P = 0.863; main effect chamber: F2,40 = 30.28, P < 0.001; three-way ANOVA with Newman-Keuls post hoc test). (d-e) 6-OHDA did not affect the morphology of VGluT2 axon terminals (AT) derived from the VTA. Electron micrographs showing the detection of PV (gold particles; blue arrowheads) in dendrites that establish asymmetric synapses (green arrows) with terminals coexpressing mCherry (scattered dark material) and VGluT2 (gold particles; green arrowheads) derived from VTA VGluT2-neurons. (d) A PV-dendrite establishing asymmetric synapses with two VGluT2-mesoaccumbens terminals (AT1 and AT2) (e) A PV-dendrite establishing asymmetric synapses with a VGluT2-mesoaccumbens terminal and containing a long mitochondrion (4.2 µm in length).
Supplementary Figure 6 Photostimulation of nAcc mShell fibers from VTA VGAT neurons does not produce rewarding or aversive effects in place-conditioning tests.
(a) Diagram of virus injection in VTA of VGAT-ires-Cre mice, and nAcc-mShell photostimulation of mesoaccumbens VGAT-fibers. (b) Detection of eYFP (green) under the control of the vgat-promoter within the VTA containing TH (red). (c-e) VTA VGAT-inputs in the nAcc are infrequent. (f) Real-time place conditioning procedure timeline. The nAcc-mShell photostimulation of mesoaccumbens VGAT-fibers was available during conditioning day 1 and day 2. (g) Photostimulation did not have rewarding or aversive effects in real-time place conditioning test. VGAT-ChR2-eYFP mice (n = 6) and VGAT-eYFP mice (n = 6) spent similar time in both chambers during both conditioning and test days (main effect group: F1,10 = 0.95, P = 0.353; group × day × chamber interaction: F6,60 = 0.12, P = 0.993; three-way ANOVA). Relative time spent in each chamber is represented as mean + SEM. Blue rectangles indicate photostimulation available in the photostimulation-paired chamber. (h) Photostimulation has no effects on distance traveled by VGAT-ChR2-eYFP mice in the conditioning chambers on conditioning day (main effect group: F1,10 = 0.831, P = 0.385; two-way ANOVA). The average travel distances for each group of mice are represented as mean + SEM.
Supplementary Figure 7 Photostimulation of nAcc mShell fibers from VTA VGluT2 neurons induces c-Fos expression in PV interneurons, but not in NOS or ChAT interneurons.
(a-d) c-Fos expression was detected in nAcc mShell of photostimulated mice. At high magnification, the PV-immunoreactive neurons coexpressed c-Fos in VGluT2-ChR2-eYFP mice (arrows in b), but not in VGluT2-eYFP mice (arrowheads in d). aca, anterior commissure anterior part; LV, lateral ventricle; Core, nucleus accumbens core; mShell, nucleus accumbens medial shell. (e-h) Photostimulation of VGluT2 inputs in nAcc mShell failed to induce c-Fos expression in NOS neurons (double arrowheads in f), or ChAT neurons (double arrows in h).
Supplementary Figure 8 PV interneurons after 20-Hz nAcc mShell photostimulation of VGluT2 mesoaccumbens fibers.
(a) Response of a PV-fast-spiking-interneuron to current injections (from −50 pA to +350 pA) (left). PV-fast-spiking interneuron fired at a rate of 25 Hz after injection of 250 pA (bottom right), 55 Hz after injection of 300 pA (middle right), and 80 Hz after injection of 350 pA (upper right). (b) EPSCs recorded in a PV-fast-spiking-interneuron after photostimulation of VGluT2-mesoaccumbens fibers at 1 Hz (top left), 10 Hz (top right), 20 Hz (bottom left) or 30 Hz (bottom right). Individual trials are shown in gray and averaged EPSCs are shown in black.
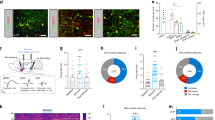
Supplementary Figure 9 nAcc mShell photostimulation of VGluT2 mesoaccumbens fibers evokes local polysynaptic GABA release.
(a) Response of a medium spiny neuron (MSN) to current steps injection (from −60 pA to + 100 pA). (b) EPSCs recorded at −70 mV in a MSN in response to nAcc-mShell photostimulation of VGluT2-mesoaccumbens fibers, before (red) or after bath application of bicuculline (10 µM; blue) or bicuculline (10 µM) + CNQX (10 µM; gray) (control = −35.78 ± 9.06 pA; bicuculline = −36.71 ± 11.04 pA; bicuculline + CNQX = −1.98 ± 0.36 pA; F2,41 = 10.83, *P = 0.0004 repeated measures ANOVA, post hoc Dunnett’s multiple comparison test; n = 14 cells from 8 mice), error bars correspond to SEM. (c) EPSCs and IPSCs recorded at −45 mV in a MSN in response to nAcc-mShell photostimulation of VGluT2-mesoaccumbens fibers, before (red) or after bath application of bicuculline (10 µM; blue) or bicuculline (10 µM) + CNQX (10 µM; gray) (EPSCs control = −35.71 ± 12.73 pA; EPSCs bicuculline = −41.69 ± 15.61 pA; EPSCs bicuculline + CNQX = −3.34 ± 1.09 pA; F2,20 = 3.793, P = 0.0152 repeated measures ANOVA, post hoc Dunnett’s multiple comparison test; IPSCs control = 28.01 ± 10.57 pA; IPSCs bicuculline = 0.51 ± 1.20 pA; IPSCs Bicuculline + CNQX = 0.38 ± 1.05 pA; F2,20 = 0.87, *P = 0.01 repeated measures ANOVA, post hoc Dunnett’s multiple comparison test, n = 7 cells from 5 mice) error bars correspond to SEM. (d) Representative traces from an MSN recording demonstrating an EPSC-IPSC response following nAcc-mShell photostimulation of VGluT2-mesoaccumbens fibers. Note the IPSC was reversibly eliminated by bicuculline (blue) whereas CNQX eliminated both the EPSC and IPSC (gray). (e) Schematic representation of direct excitatory monosynaptic VGluT2-mesoaccumbens inputs onto MSNs in the nAcc mShell (left) or polysynaptic inhibition of MSNs via mesoaccumbens-driven glutamate release onto PV-interneurons (right).
Supplementary Figure 10 Photostimulation of nAcc mShell PV interneurons results in place aversion.
(a) Diagram of virus injection and optical probe in nAcc-mShell of PV::Cre mice. (b) nAcc expression of eYFP (green) under the control of the pv-promoter in PV::Cre mice. (c and d) Detection of eYFP (green) in neurons coexpressing PV (red). (e) Real-time place conditioning procedure timeline. The PV-ChR2-eYFP and PV-eYFP mice received nAcc mShell photostimulation during conditioning days (D1 and D2). (f) During conditioning days, PV-ChR2-eYFP mice (n = 6) but not PV-eYFP (n = 6) spent less time in the chamber where nAcc mShell photostimulation was given. Blue rectangles indicate photostimulation available in the photostimulation-associated chamber. On the test day, PV-ChR2-eYFP mice still spent less time in the chamber previously associated with photostimulation. PV-ChR2-eYFP mice showed aversion to the photostimulation-associated chamber on both conditioning days and test day (main effect chamber: F2,20 = 7.88, P = 0.003; group × day × chamber interaction: F6,60= 0.85, P = 0.54; three-way ANOVA, * P < 0.05, Newman-Keuls post hoc test). (g) Photostimulation had no differences in travel distance between PV-ChR2-eYFP and PV-eYFP mice. Data are represented as mean + SEM.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 1 and 2 (PDF 5404 kb)
Source data
Rights and permissions
About this article
Cite this article
Qi, J., Zhang, S., Wang, HL. et al. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat Neurosci 19, 725–733 (2016). https://doi.org/10.1038/nn.4281
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4281
This article is cited by
-
Maturation of nucleus accumbens synaptic transmission signals a critical period for the rescue of social deficits in a mouse model of autism spectrum disorder
Molecular Brain (2023)
-
D1 receptor-expressing neurons in ventral tegmental area alleviate mouse anxiety-like behaviors via glutamatergic projection to lateral septum
Molecular Psychiatry (2023)
-
Ventral tegmental area glutamate neurons establish a mu-opioid receptor gated circuit to mesolimbic dopamine neurons and regulate opioid-seeking behavior
Neuropsychopharmacology (2023)
-
Inhibition of ventral tegmental area projections to the nucleus accumbens shell increases premature responding in the five-choice serial reaction time task in rats
Brain Structure and Function (2023)
-
Neuropeptides Modulate Feeding via the Dopamine Reward Pathway
Neurochemical Research (2023)