Abstract
Resilience to aversive events has a central role in determining whether stress leads to the development of depression. mGluR5 has been implicated in the pathophysiology of depression, but the effect of mGluR5 activity on stress resilience remains unexplored. We found that mGluR5−/− (also known as Grm5−/−) mice displayed more depression-like behaviors (for example, learned helplessness, social withdrawal and anhedonia) than control mice following exposure to various stressful stimuli. Lentiviral 'rescue' of mGluR5 in the nucleus accumbens (NAc) decreased these depression-like behaviors in mGluR5−/− mice. In the NAc, ΔFosB, whose induction promotes stress resilience, failed to be upregulated by stress in mGluR5−/− mice. Notably, targeted pharmacological activation of mGluR5 in the NAc increased ΔFosB expression. Our findings point to an essential role for mGluR5 in promoting stress resilience and suggest that a defect in mGluR5-mediated signaling in the NAc may represent an endophenotype for stress-induced depression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kendler, K.S., Karkowski, L.M. & Prescott, C.A. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 156, 837–841 (1999).
Nestler, E.J. et al. Neurobiology of depression. Neuron 34, 13–25 (2002).
Russo, S.J., Murrough, J.W., Han, M.H., Charney, D.S. & Nestler, E.J. Neurobiology of resilience. Nat. Neurosci. 15, 1475–1484 (2012).
Southwick, S.M. & Charney, D.S. The science of resilience: implications for the prevention and treatment of depression. Science 338, 79–82 (2012).
Feder, A., Nestler, E.J. & Charney, D.S. Psychobiology and molecular genetics of resilience. Nat. Rev. Neurosci. 10, 446–457 (2009).
Franklin, T.B., Saab, B.J. & Mansuy, I.M. Neural mechanisms of stress resilience and vulnerability. Neuron 75, 747–761 (2012).
Barrot, M. et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc. Natl. Acad. Sci. USA 99, 11435–11440 (2002).
Perrotti, L.I. et al. Induction of deltaFosB in reward-related brain structures after chronic stress. J. Neurosci. 24, 10594–10602 (2004).
Vialou, V. et al. Serum response factor promotes resilience to chronic social stress through the induction of DeltaFosB. J. Neurosci. 30, 14585–14592 (2010).
Vialou, V. et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat. Neurosci. 13, 745–752 (2010).
Niswender, C.M. & Conn, P.J. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322 (2010).
Kim, C.H., Lee, J., Lee, J.Y. & Roche, K.W. Metabotropic glutamate receptors: phosphorylation and receptor signaling. J. Neurosci. Res. 86, 1–10 (2008).
Nicoletti, F. et al. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60, 1017–1041 (2011).
Chiamulera, C. et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat. Neurosci. 4, 873–874 (2001).
Li, X., Need, A.B., Baez, M. & Witkin, J.M. Metabotropic glutamate 5 receptor antagonism is associated with antidepressant-like effects in mice. J. Pharmacol. Exp. Ther. 319, 254–259 (2006).
Tatarczyńska, E. et al. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br. J. Pharmacol. 132, 1423–1430 (2001).
Kovacčevic´, T., Skelin, I., Minuzzi, L., Rosa-Neto, P. & Diksic, M. Reduced metabotropic glutamate receptor 5 in the Flinders Sensitive Line of rats, an animal model of depression: an autoradiographic study. Brain Res. Bull. 87, 406–412 (2012).
Deschwanden, A. et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am. J. Psychiatry 168, 727–734 (2011).
Belozertseva, I.V., Kos, T., Popik, P., Danysz, W. & Bespalov, A.Y. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur. Neuropsychopharmacol. 17, 172–179 (2007).
Cryan, J.F. & Slattery, D.A. Animal models of mood disorders: Recent developments. Curr. Opin. Psychiatry 20, 1–7 (2007).
Lim, B.K., Huang, K.W., Grueter, B.A., Rothwell, P.E. & Malenka, R.C. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189 (2012).
Krishnan, V. et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404 (2007).
Nestler, E.J. & Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 13, 1161–1169 (2010).
Misra, R.P. et al. L-type voltage-sensitive calcium channel activation stimulates gene expression by a serum response factor-dependent pathway. J. Biol. Chem. 269, 25483–25493 (1994).
Janknecht, R., Hipskind, R.A., Houthaeve, T., Nordheim, A. & Stunnenberg, H.G. Identification of multiple SRF N-terminal phosphorylation sites affecting DNA binding properties. EMBO J. 11, 1045–1054 (1992).
Ulery, P.G., Rudenko, G. & Nestler, E.J. Regulation of DeltaFosB stability by phosphorylation. J. Neurosci. 26, 5131–5142 (2006).
Hughes, Z.A. et al. Negative allosteric modulation of metabotropic glutamate receptor 5 results in broad spectrum activity relevant to treatment resistant depression. Neuropharmacology 66, 202–214 (2013).
Donahue, R.J., Muschamp, J.W., Russo, S.J., Nestler, E.J. & Carlezon, W.A. Jr. Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol. Psychiatry 76, 550–558 (2014).
Nestler, E.J. & Fos, B. A transcriptional regulator of stress and antidepressant responses. Eur. J. Pharmacol. 753, 66–72 (2014).
Britt, J.P. et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76, 790–803 (2012).
Jia, Z. et al. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn. Mem. 5, 331–343 (1998).
Bougarel, L., Guitton, J., Zimmer, L., Vaugeois, J.M. & El Yacoubi, M. Behavior of a genetic mouse model of depression in the learned helplessness paradigm. Psychopharmacology (Berl.) 215, 595–605 (2011).
Chourbaji, S. et al. Learned helplessness: validity and reliability of depressive-like states in mice. Brain Res. Brain Res. Protoc. 16, 70–78 (2005).
Golden, S.A., Covington, H.E. III., Berton, O. & Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 6, 1183–1191 (2011).
Guo, M. et al. Forebrain glutamatergic neurons mediate leptin action on depression-like behaviors and synaptic depression. Transl. Psychiatry 2, e83 (2012).
Iñiguez, S.D. et al. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J. Neurosci. 30, 7652–7663 (2010).
Porsolt, R.D., Brossard, G., Hautbois, C. & Roux, S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 8, 8.10A (2001).
Kutner, R.H., Zhang, X.Y. & Reiser, J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 4, 495–505 (2009).
Fowler, C.D., Lu, Q., Johnson, P.M., Marks, M.J. & Kenny, P.J. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471, 597–601 (2011).
Ko, S.J. et al. PKC phosphorylation regulates mGluR5 trafficking by enhancing binding of Siah-1A. J. Neurosci. 32, 16391–16401 (2012).
Berton, O. et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868 (2006).
Bruchas, M.R. et al. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron 71, 498–511 (2011).
Acknowledgements
We thank the Yonsei-Carl Zeiss Advanced Imaging Center for technical assistance. We thank G. Panagiotakos for her careful and critical reading. This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2014R1A2A1A11051372 & no. 2007-0056092 to C.H.K., no. 2013054004 to D.G.K.), the DGIST R&D Program of the Ministry of Science, ICT and Future Planning (14-BD-16 to C.H.K.), and a faculty research grant from Yonsei University College of Medicine for 2012 (no. 6-2012-0018 to D.G.K.).
Author information
Authors and Affiliations
Contributions
S.S., O.K., C.H.K. and D.G.K. designed the experiments and analyzed the data. C.H.K. and D.G.K. prepared the manuscript. S.S., O.K., S.O. and J.C. performed the experiments. J.I.K. and S.K. provided scientific input and helped edit the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
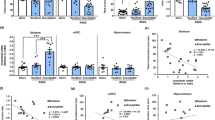
Supplementary Figure 1 Basic characteristics of mGluR5−/− mice.
(a) At 16 week of age, mGluR5−/− mice showed reduced body weights compared with wild-type mice (n = 14 mice for mGluR5+/+ and 12 for each mGluR5+/– and mGluR5−/−, respectively). One-way ANOVA (F(2,35) = 6.661, P = 0.004) followed by Bonferroni post hoc test for multiple comparisons; **P = 0.003 compared with wild-type mice. (b) Locomotor activity was monitored for 15 min and analyzed in 3 min bins (n = 10 mice for each mGluR5+/+ and mGluR5+/– and 11 for mGluR5−/−, respectively). (c) Total distance traveled during the 15 min test period. No significant difference in locomotor activity was observed between genotypes. (d) Rearing counts (vertical activity) were unchanged in mGluR5+/– and mGluR5−/− mice (n = 8 mice for mGluR5+/+ and 7 for each mGluR5+/– and mGluR5−/−, respectively). Data are expressed as the mean ± SEM. (e) Western blot analysis of whole brain lysates and crude synaptosomal fractions from mGluR5+/+, mGluR5+/–, and mGluR5−/− mice. mGluR5−/− mice showed normal levels of other subtypes of mGluRs (mGluR1, mGluR2/3, and mGluR7), ionotropic glutamate receptors (GluR1, GluR2, NR2A, and NR2B), and synaptic scaffold proteins (PSD-95, PICK1, Pan-MAGUK, Chapsyn-110, and SAP-102). The experiment was successfully repeated three times. Full-length blots are presented in Supplementary Figure 9.
Supplementary Figure 2 mGluR5−/− mice exhibit depression-like behaviors in two-day FST and TST.
(a,b) FST over two consecutive days. Immobility (a) and struggling time (b) were measured during the last 4 min of the 6-min swim test period on day 1 and day 2 respectively (n = 8 mice for mGluR5+/+ and 7 for mGluR5–/–). Two-sided t-test (day2 mGluR5+/+ vs mGluR5–/–, t(13) = –2.504 in a and t(13) = 2.899 in b); P < 0.05 compared with wild-type mice on day 2. (c) Tail suspension test (TST) over two consecutive days. Immobility time was measured during the last 4 min of the 6-min suspension test period on day 1 and day 2 respectively. Two-sided t-test (day2 mGluR5+/+ vs mGluR5–/–, t(10) = –2.236); *P = 0.049 compared with wild-type mice on day 2. (d,e) Time spent immobile per 1-min interval within the 6-min TST session on day 1 (d) and day 2 (e) (n = 7 mice for mGluR5+/+ and 5 for mGluR5–/–). Two-way RM ANOVA (in e, genotype × time interaction F(5,50) = 1.552, P = 0.191) followed by Bonferroni post hoc test for multiple comparisons; *P < 0.05 compared with wild-type mice at each time point. Data are expressed as the mean ± SEM.
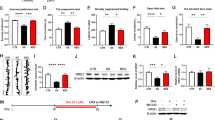
Supplementary Figure 3 Validation of mGluR5-expressing lentivirus and verification of correct injection site in the NAc shell and core.
(a) Diagram of the lentiviral vector used to rescue mGluR5. (b) Validation of the mGluR5-lentivirus. Western blot analysis of mGluR5 expression in HEK293FT cells 6 days after transduction of the control-LV (lentivirus) or mGluR5-LV with low, medium, and high concentrations. Full-length blots are presented in Supplementary Figure 10. The experiment was successfully repeated two times. (c) Immunohistochemistry of mGluR5 overlapping ZsGreen in the NAc shell of mice injected with mGluR5-LV. Scale bars, 50 μm. The experiment was successfully repeated three times. (d) Immunofluorescence of ZsGreen in the NAc shell (left) and NAc core (right) of mice injected with control-LV. ac, anterior commissure; AcbC, nucleus accumbens core; Acbsh, nucleus accumbens shell. Scale bars, 100 μm. The experiment was successfully repeated three times.
Supplementary Figure 4 ‘Rescue’ of mGluR5 in the NAc shell normalizes sucrose preference.
(a) Localized expression of mGluR5 within the NAc shell reversed the 3-day restraint stress-induced anhedonia observed in mGluR5−/− mice (n = 9, 5, and 6 mice for WT-control LV, KO-control LV and KO-mGluR5 LV, respectively). Two-way RM ANOVA (group × stress interaction F(2,17) = 9.042, P = 0.002) followed by Fisher LSD post hoc test for multiple comparisons; ##P = 0.001 compared with the WT-control LV following restraint stress; ††P = 0.004 compared with the respective pre-stressed mice; §P = 0.028 compared with the KO-control LV following restraint stress. (b) Rescue of mGluR5 in the NAc shell did not alter total liquid intake (water plus sucrose solution) before and after the 3-day restraint stress. Data are expressed as the mean ± SEM.
Supplementary Figure 5 Learned helplessness level after electric foot shock is correlated with mGluR5 level in NAc shell.
(a) Susceptible mice showed higher mean escape latency compared to non-defeat control mice, while resilient mice did not (n = 5, 8, and 12 mice for unshocked, susceptible, and resilient group, respectively). One-way ANOVA (F(2,22) = 72.955, P < 0.001) followed by Fisher LSD post hoc test for multiple comparisons; ***P < 0.001 compared with control group; †††P < 0.001 compared with resilient group. (b) Mean escape latency during 5-trial blocks. Two-way RM ANOVA (group × number of block interaction F(10,110) = 1.440, P = 0.172) followed by Fisher LSD post hoc test for multiple comparisons; ***P < 0.001 compared with unshocked mice; †††P < 0.001 compared with resilient group at each trial block. (c) Western blot images and quantification analysis of mGluR5 level in the NAc of susceptible mice following learned helplessness paradigm showed lower level compared to that of control mice, while resilient mice did not. One-way ANOVA (F(2,22) = 4.042, P = 0.032) followed by Fisher LSD post hoc test for multiple comparisons; *P = 0.046 compared with control group; †P = 0.014 compared with resilient group. Data are expressed as the mean ± SEM. Full-length blots are presented in Supplementary Figure 10. (d) There was a significant correlation between mean escape latency and mGluR5 level in the NAc; Pearson correlation.
Supplementary Figure 6 Lentiviral ΔFosB expression in the NAc shell prevents social avoidance in mGluR5−/− mice following a 3-day social defeat stress.
(a) Diagram of the lentiviral vector used to express ΔFosB. Scale bar, 50 μm. For each mouse used, precise delivery of the virus was confirmed by visualizing ZsGreen1-expressing cells. (b) Validation of the ΔFosB-lentivirus. Western blot analysis of ΔFosB expression in HEK293FT cells 6 days after transduction of the control-LV (lentivirus) or ΔFosB-LV. Full-length blots are presented in Supplementary Figure 10. (c,d) mGluR5−/− mice that received ΔFosB LV in the NAc shell exhibited times spent in the interaction zone (c) and the corner zone (d) that were comparable to those of control LV-injected mGluR5+/+ mice (n = 4, 6, and 9 mice for WT-control LV, KO-control LV and KO-ΔFosB LV, respectively). Two-way RM ANOVA (in c, group × target interaction F(2,16) = 3.821, P = 0.044) followed by Bonferroni post hoc test for multiple comparisons; #P = 0.012, ##P = 0.006 compared with the WT-control LV in the presence of a target; †P = 0.046, ††P = 0.004 compared with the respective no-target conditions; §P = 0.028, §§P = 0.002 compared with the KO-control LV in the presence of a target. Data are expressed as the mean ± SEM.
Supplementary Figure 7 Phospho-SRF (Ser103) mediates the induction of ΔFosB by mGluR5 after 3-day social defeat stress.
Representative double immunohistochemistry of the NAc shell of wild-type and mGluR5–/– mice following a 3-day social defeat stress (lower 2 rows) or not (upper 2 rows). Scale bars, 50 μm. The experiment was successfully repeated three times.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 2824 kb)
Rights and permissions
About this article
Cite this article
Shin, S., Kwon, O., Kang, J. et al. mGluR5 in the nucleus accumbens is critical for promoting resilience to chronic stress. Nat Neurosci 18, 1017–1024 (2015). https://doi.org/10.1038/nn.4028
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4028
This article is cited by
-
Examining sex differences in responses to footshock stress and the role of the metabotropic glutamate receptor 5: an [18F]FPEB and positron emission tomography study in rats
Neuropsychopharmacology (2023)
-
A sex-specific genome-wide association study of depression phenotypes in UK Biobank
Molecular Psychiatry (2023)
-
Lateral hypothalamic proenkephalin neurons drive threat-induced overeating associated with a negative emotional state
Nature Communications (2023)
-
LHPP, a risk factor for major depressive disorder, regulates stress-induced depression-like behaviors through its histidine phosphatase activity
Molecular Psychiatry (2023)
-
Amygdala Metabotropic Glutamate Receptor 1 Influences Synaptic Transmission to Participate in Fentanyl-Induced Hyperalgesia in Rats
Cellular and Molecular Neurobiology (2023)