Abstract
An essential feature of vertebrate neural development is ensheathment of axons with myelin, an insulating membrane formed by oligodendrocytes. Not all axons are myelinated, but mechanisms directing myelination of specific axons are unknown. Using zebrafish, we found that activity-dependent secretion stabilized myelin sheath formation on select axons. When VAMP2-dependent exocytosis was silenced in single axons, oligodendrocytes preferentially ensheathed neighboring axons. Nascent sheaths formed on silenced axons were shorter in length, but when activity of neighboring axons was also suppressed, inhibition of sheath growth was relieved. Using in vivo time-lapse microscopy, we found that only 25% of oligodendrocyte processes that initiated axon wrapping were stabilized during normal development and that initiation did not require activity. Instead, oligodendrocyte processes wrapping silenced axons retracted more frequently. We propose that axon selection for myelination results from excessive and indiscriminate initiation of wrapping followed by refinement that is biased by activity-dependent secretion from axons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sturrock, R.R. Myelination of the mouse corpus callosum. Neuropathol. Appl. Neurobiol. 6, 415–420 (1980).
Rosenberg, S.S., Kelland, E.E., Tokar, E., de la Torre, A.R. & Chan, J.R. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc. Natl. Acad. Sci. USA 105, 14662–14667 (2008).
Lee, S. et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9, 917–922 (2012).
Gyllensten, L. & Malmfors, T. Myelinization of the optic nerve and its dependence on visual function–a quantitative investigation in mice. J. Embryol. Exp. Morphol. 11, 255–266 (1963).
Tauber, H., Waehneldt, T.V. & Neuhoff, V. Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci. Lett. 16, 235–238 (1980).
Demerens, C. et al. Induction of myelination in the central nervous system by electrical activity. Proc. Natl. Acad. Sci. USA 93, 9887–9892 (1996).
Wake, H., Lee, P.R. & Fields, R.D. Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651 (2011).
Barres, B.A. & Raff, M.C. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260 (1993).
Gibson, E.M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014).
Kukley, M., Capetillo-Zarate, E. & Dietrich, D. Vesicular glutamate release from axons in white matter. Nat. Neurosci. 10, 311–320 (2007).
Ziskin, J.L., Nishiyama, A., Rubio, M., Fukaya, M. & Bergles, D.E. Vesicular release of glutamate from unmyelinated axons in white matter. Nat. Neurosci. 10, 321–330 (2007).
Ng, B.K.B., Chen, L.L., Mandemakers, W.W., Cosgaya, J.M.J. & Chan, J.R.J. Anterograde transport and secretion of brain-derived neurotrophic factor along sensory axons promote Schwann cell myelination. J. Neurosci. 27, 7597–7603 (2007).
Káradóttir, R. & Attwell, D. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience 145, 1426–1438 (2007).
Makinodan, M., Rosen, K.M., Ito, S. & Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 (2012).
Liu, J. et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623 (2012).
Ben Fredj, N. et al. Synaptic activity and activity-dependent competition regulates axon arbor maturation, growth arrest, and territory in the retinotectal projection. J. Neurosci. 30, 10939–10951 (2010).
Agetsuma, M. et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat. Neurosci. 13, 1354–1356 (2010).
Hua, J.Y., Smear, M.C., Baier, H. & Smith, S.J. Regulation of axon growth in vivo by activity-based competition. Nature 434, 1022–1026 (2005).
McLaughlin, T., Torborg, C.L., Feller, M.B. & O'Leary, D.D.M. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 40, 1147–1160 (2003).
Nicol, X. et al. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat. Neurosci. 10, 340–347 (2007).
Plazas, P.V., Nicol, X. & Spitzer, N.C. Activity-dependent competition regulates motor neuron axon pathfinding via PlexinA3. Proc. Natl. Acad. Sci. USA 110, 1524–1529 (2013).
Kishore, S. & Fetcho, J.R. Homeostatic regulation of dendritic dynamics in a motor map in vivo. Nat. Commun. 4, 2086 (2013).
Umeda, K. et al. Targeted expression of a chimeric channelrhodopsin in zebrafish under regulation of Gal4-UAS system. Neurosci. Res. 75, 69–75 (2013).
Mensch, S. et al. Synaptic vesicle release regulates the number of myelin sheaths made by individual oligodendrocytes in vivo. Nat. Neurosci. doi:10.1038/nn.3991 (6 April 2015).10.1038/nn.3991
Czopka, T., ffrench-Constant, C. & Lyons, D.A. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev. Cell 25, 599–609 (2013).
Colello, R.J., Pott, U. & Schwab, M.E. The role of oligodendrocytes and myelin on axon maturation in the developing rat retinofugal pathway. J. Neurosci. 14, 2594–2605 (1994).
Sánchez, I., Hassinger, L., Paskevich, P.A., Shine, H.D. & Nixon, R.A. Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. J. Neurosci. 16, 5095–5105 (1996).
Almeida, R.G. & Lyons, D.A. On the resemblance of synapse formation and CNS myelination. Neuroscience 276, 98–108 (2014).
McKenzie, I.A. et al. Motor skill learning requires active central myelination. Science 346, 318–322 (2014).
Shrager, P. & Novakovic, S.D. Control of myelination, axonal growth, and synapse formation in spinal cord explants by ion channels and electrical activity. Brain Res. Dev. Brain Res. 88, 68–78 (1995).
De Biase, L.M. et al. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J. Neurosci. 31, 12650–12662 (2011).
Káradóttir, R., Hamilton, N.B., Bakiri, Y. & Attwell, D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat. Neurosci. 11, 450–456 (2008).
Chittajallu, R., Aguirre, A. & Gallo, V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J. Physiol. (Lond.) 561, 109–122 (2004).
Fields, R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 31, 361–370 (2008).
Nechiporuk, A., Linbo, T., Poss, K.D. & Raible, D.W. Specification of epibranchial placodes in zebrafish. Development 134, 611–623 (2007).
Heap, L.A., Goh, C.C., Kassahn, K.S. & Scott, E.K. Cerebellar output in zebrafish: an analysis of spatial patterns and topography in eurydendroid cell projections. Front Neural Circuits 7, 53 (2013).
Kucenas, S. et al. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat. Neurosci. 11, 143–151 (2008).
Jung, S.-H. et al. Visualization of myelination in GFP-transgenic zebrafish. Dev. Dyn. 239, 592–597 (2010).
Kwan, K.M. et al. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 (2007).
Mo, W. & Nicolson, T. Both pre- and postsynaptic activity of Nsf prevents degeneration of hair-cell synapses. PLoS ONE 6, e27146 (2011).
Kucenas, S., Wang, W.-D., Knapik, E.W. & Appel, B. A selective glial barrier at motor axon exit points prevents oligodendrocyte migration from the spinal cord. J. Neurosci. 29, 15187–15194 (2009).
Park, H.-C., Boyce, J., Shin, J. & Appel, B. Oligodendrocyte specification in zebrafish requires notch-regulated cyclin-dependent kinase inhibitor function. J. Neurosci. 25, 6836–6844 (2005).
Acknowledgements
We are grateful to A.J. Treichel (Winona State University) for help with image analysis. We thank A. Nechiporuk (Oregon Health Sciences University) for Tg(phox2b:EGFP) zebrafish, H. Yawo (Tohoku University) and the National BioResource Zebrafish Project for Tg(UAS:ChRWR-EGFP) zebrafish, M. Meyer (King's College London) for the pCS2-TeNT-EGFP plasmid and J. Fetcho (Cornell University) for the UAS:Kir2.1-2A-EGFP plasmid. We thank M. Goll (Memorial Sloan-Kettering Cancer Center), M. Nonet (Washington University), C.-B. Chien (University of Utah) and K. Kwan (University of Utah) for Gateway plasmids. For critical feedback on the project, we thank M. Rasband, J. Triplett, S. Henle, A. Ribera and W. Macklin, and all members of the Appel, Ribera and Macklin laboratories. We also thank J. Costabile and the University of Colorado Anschutz Medical Campus Optogenetics Core Facility for assistance with UAS:ChRWR-EGFP experiments. This work was supported by US National Institutes of Health (NIH) grant R01 NS046668 and a gift from the Gates Frontiers Fund to B.A., a National Multiple Sclerosis Postdoctoral Fellowship (FG 2024-A-1) and NIH (NIMH) fellowship T32 MN015442 to J.H.H. and NIH (NCI) fellowship T32 5T32CA08208613 to A.M.R. The University of Colorado Anschutz Medical Campus Zebrafish Core Facility is supported by NIH grant P30 NS048154. All DNA plasmids and transgenic zebrafish used in this study are available by request.
Author information
Authors and Affiliations
Contributions
J.H.H. and B.A. conceived the project. J.H.H. generated all new transgenic zebrafish lines and performed fluorescence microscopy experiments. A.M.R. cloned the zebrafish phox2b promoter and performed veratridine and Channelrhodopsin experiments. R.S. performed electron microscopy experiments and assisted with cell count experiments. E.K.S. produced the Tg(UAS:Syp-EGFP) transgenic line. J.H.H. wrote and B.A. edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
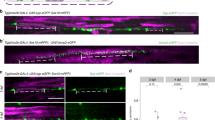
Supplementary Figure 1 Myelin-fated axons are marked by Tg(phox2b:EGFP) and Tg(phox2b:GAL4).
(a) Fluorescence images show reporter expression in a Tg(phox2b:EGFP); Tg(sox10:mRFP) larva. (b) Confocal images show phox2B:EGFP and sox10:mRFP reporter expression in the spinal cord. Images are lateral views. Scale bar, 5 µm. (c) Transverse section of a 7 dpf Tg(phox2b:EGFP) larva fixed and immunolabeled for Myelin basic protein (MBP) to show the localization of myelin sheaths. Inset (right) shows a higher magnification view of phox2b:EGFP+ axons, which are surrounded by MBP immunofluorescence (yellow arrowheads). Scale bar, 1 µm. (d) Confocal images show co-expression in Tg(phox2b:EGFP) and Tg(phox2b:GAL4) reporter lines. The Tg(phox2b:GAL4) driver activates the Tg(UAS:mCherry-CaaX) reporter, which results in mosaic mCherry expression in a subset of GAL4+ axons due to UAS silencing (see Methods). The arrowhead points to a mCherry+ axon co-expressing EGFP. Scale bar, 5 µm.
Supplementary Figure 2 Spinal cord cell counts in TTX-treated larvae.
Fluorescence images show Tg(olig2:EGFP) and Sox10 immunofluorescence in control and TTX-treated larvae. Images are transverse spinal cord sections, shown as z-projections, with dorsal up. Arrowheads point to olig2+ Sox10+ OPCs. Scale bar, 10 µm. (b) Quantification of olig2+ Sox10+ cell counts show the mean number of double positive cells per transverse section. Error bars show SEM; P = 0.3503, t-test; n.s., not significant; n = 10 control and 15 TTX-treated larvae. (c) Fluorescence images show Tg(mbp:EGFP) reporter expression in control and TTX-treated larvae. Images are lateral view z-projections encompassing half of the spinal cord with dorsal up and anterior right. Scale bar, 10 µm. (d) Quantification of mbp+ counts show the average number of cells per spinal cord hemisegment. Error bars show s.e.m.; P = 0.7210, t-test; n.s., not significant; n = 21 control and 21 TTX-treated larvae.
Supplementary Figure 3 Selection of UAS:ChRWR-EGFP axons.
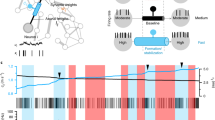
(a) Quantification of the percentage of axons selected for myelination in control larvae (ChRWR-EGFP+, no light stimulation) and ChRWR-EGFP+ larvae treated with 473 nm light (see Methods). The number of axons selected for myelination and the total number of axons analyzed is indicated. c) Quantitative measurements show the wrapping efficiency of phox2b+ axons in control and 473 nm light-treated larvae. Data are expressed as the percent of total axon length ensheathed at the time of imaging. Error bars show s.e.m.; P = 0.9383, t-test; n.s., not significant; data shown are derived from 12 control and 15 light-treated larvae.
Supplementary Figure 4 Characterization of Tg(neurod1:TeNT-EGFP) larvae.
(a) Transmitted light (left) and fluorescence images (right) show TeNT-EGFP expression in a 4 dpf Tg(neurod1:TeNT-EGFP) larva. (b) Representative confocal images show lateral views of a 3 dpf Tg(neurod1:TeNT-EGFP), Tg(sox10:TagRFP) larvae. The left panel shows a projection of z-planes encompassing the entire spinal cord, and the right panel shows a single z-plane. Arrowheads point to oligodendrocytes marked by sox10:TagRFP in the ventral spinal cord. Note that TeNT-EGFP is broadly expressed, but is not found in sox10:TagRFP+ oligodendrocytes (arrowheads). Scale bar, 5 µm. See Supplementary Video 4, which shows the complete paralysis of Tg(neurod1:TeNT-EGFP) larvae.
Supplementary Figure 5 Sheath number, but not sheath length, is reduced in Tg(neurod1:TeNT-EGFP) larvae.
(a) Representative confocal images of single oligodendrocytes labeled by Tg(mbp:GAL4-VP16, cmlc2:Cerulean); UAS:mCherry-CaaX expression. Images are spinal cord lateral views of 5 dpf larvae. Scale bar, 5 µm. (b) Quantitative measurements show the average number of sheaths per individual oligodendrocyte. P = 0.0043, t-test. (c) Quantitative measurements show the average sheath length for individual oligodendrocytes. P = 0.2023, t-test. For (b-c), n = 13 sibling and 13 Tg(neurod1:TeNT-EGFP) larvae. (d) Summary of sox10+ and mbp+ cell counts in control (sibling) and Tg(neurod1:TeNT-EGFP) larvae. n = 13, 9, 7, 6, 16, and 13 larvae. P < 0.0001 (left comparison), P < 0.0001 (center comparison), P = 0.0011 (right comparison). For (b-d), error bars show SEM, t-test, **P < 0.01, ***P < 0.001; n.s., not significant.
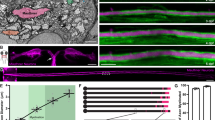
Supplementary Figure 6 Comparison of axon diameter in wild-type and Tg(neurod1:TeNT-EGFP) larvae.
(a) Transmission electron microscope images of ventral spinal cord, including Mauthner (M) axons. Images are transverse sections of 3 dpf larvae; dorsal axis toward the top and medial to the right. Scale bar, 1 µm. (b) Quantification of axon diameters shows a similar distribution in siblings and Tg(neurod1:TeNT-EGFP) larvae. The size diameter distributions were not statistically significant; P = 0.41, Kolmogorov-Smirnov test. (c) Average axon diameter for data shown in (b). The average diameter was not significantly different between groups; P = 0.98, t-test. For (a-c), n = 3 wild-type larvae (204 axons) and 3 Tg(neurod1:TeNT-EGFP) larvae (185 axons); error bars show s.e.m.
Supplementary Figure 7 Time course of myelin sheath initiations and retractions in Tg(neurod1:TeNT-EGFP) larvae.
(a) Confocal images of a single oligodendrocyte marked by UAS:mCherry-CaaX expression in a Tg(sox10:GAL4-VP16, myl7:Cerulean); Tg(neurod1:TeNT-EGFP) larva. Images are lateral view of the spinal cord and time is indicated as min:sec. Blue arrowheads mark initiating sheaths and red arrowheads mark failed ensheathments. Scale bar, 10 µm. (b) Summary of length measurements for sheaths shown in (a). Each line represents an individual sheath, which will either be stable throughout the time-lapse imaging (blue) or retracted (red). Note that retracted sheaths never extend greater than 10 µm. (c) Summary of sheath length measurements from time-lapse imaging showing in (A). Each point represents the maximum length of an individual sheath during the first three hours after the sheath had initiated. Sheaths that later retracted remained short, whereas sheaths that were stabilized and maintained throughout the time-lapse began extending during this initial 3 hr period. (d) Summary of nascent sheath fates during time-lapse imaging in A. Each horizontal series represents an individual nascent sheath. Stable sheaths are blue and retracting sheaths are red.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1988 kb)
Spontaneous and evoked swim behavior in control larvae.
This video shows the touch response of 3-dpf wild-type larvae (corresponding to Fig. 2). Time-lapse images are shown in real time. (MOV 879 kb)
Spontaneous and evoked swim behavior in veratridine-treated larvae.
Videos show the behavior of 3-dpf control larvae after treatment with veratridine (corresponding to Fig. 2). Note the prolonged swim after touch stimulation that terminates in seizure-like behavior (magnified in the second time-lapse series). The third time-lapse image series shows spontaneous and spastic swim movements in a veratridine-treated larva. All time-lapse images are shown in real time. (MOV 14583 kb)
Examples of Syp-EGFP+ vesicles at unmyelinated segments and ensheathment sites.
This video corresponds to Figure 4b. Time (s) is indicated at the top left. Scale bar, 2 μm. (MOV 260 kb)
Swim behavior of sibling control and Tg(neurod1:TeNT-EGFP) larvae.
This video shows swim behaviors of a wild-type control (first larva touched) and Tg(neurod1:TeNT-EGFP) larva (second larva touched). Images were captured at 0.2-s intervals and the time (min:s) is indicated at the top left. (MOV 1609 kb)
Examples of transient wrapping.
This video shows five representative examples of short-lived prospective sheaths, which correspond to data points in Figure 5c. We acquired images at 2.5-min intervals, and time (min:s) relative to the initiation of each nascent sheath is shown at the upper left. Arrows indicate sheath initiation (blue) and failure (red). (MOV 1904 kb)
Confocal time-lapse of a stable wrapping in a sibling control larva.
This video corresponds to image panel 1 in Figure 5a. Time (h:min:s) relative to the initiation of each sheath is shown at the top left. Images are lateral spinal cord views with dorsal up and anterior right. Blue arrows indicate initiation of wrapping. (MOV 5554 kb)
Confocal time-lapse of a transient wrapping in a sibling control larva.
This video corresponds to image panel 2 in Figure 5a. Time (h:min:s) relative to the initiation of each sheath is shown at the top left. Images are lateral spinal cord views with dorsal up and anterior right. Arrows indicate wrapping initiation (blue) and failure (red). (MOV 6016 kb)
Confocal time-lapse of a stable wrapping in a Tg(neurod1:TeNT-EGFP) larva.
This video corresponds to image panel 3 in Figure 5a. Time (h:min:s) relative to the initiation of each wrap is shown at the top left. Images are lateral spinal cord views with dorsal up and anterior right. Blue arrows indicate sheath initiation. (MOV 7837 kb)
Confocal time-lapse of a transient wrapping in a Tg(neurod1:TeNT-EGFP) larva.
This video corresponds to image panel 4 in Figure 5a. Time (h:min:s) relative to the initiation of each wrap is shown at the top left. Images are lateral spinal cord views with dorsal up and anterior right. Arrows indicate wrap initiation (blue) and failure (red). (MOV 2283 kb)
Confocal time-lapse of myelin sheath maintenance in a sibling control larva.
This video corresponds to the left image panel in Figure 6a. Time (h:min) relative to the onset of time-lapse imaging is shown at the top left. Images are lateral spinal cord views with dorsal up and anterior right. (MOV 2960 kb)
Confocal time-lapse of myelin sheath maintenance in a Tg(neurod1:TeNT-EGFP) larva.
This video corresponds to the right panel in Figure 6a. Time (h:min) relative to the onset of time-lapse imaging is shown at the top left. Images are lateral spinal cord views with dorsal up and anterior right. Red arrows point to retracting sheaths. (MOV 1902 kb)
Rights and permissions
About this article
Cite this article
Hines, J., Ravanelli, A., Schwindt, R. et al. Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci 18, 683–689 (2015). https://doi.org/10.1038/nn.3992
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3992
This article is cited by
-
Oligodendrocyte death initiates synchronous remyelination to restore cortical myelin patterns in mice
Nature Neuroscience (2023)
-
Adaptive and maladaptive myelination in health and disease
Nature Reviews Neurology (2022)
-
Maladaptive myelination promotes generalized epilepsy progression
Nature Neuroscience (2022)
-
CNS myelination requires VAMP2/3-mediated membrane expansion in oligodendrocytes
Nature Communications (2022)
-
Motor learning drives dynamic patterns of intermittent myelination on learning-activated axons
Nature Neuroscience (2022)