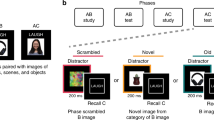
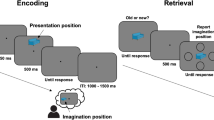
Abstract
Remembering a past experience can, surprisingly, cause forgetting. Forgetting arises when other competing traces interfere with retrieval and inhibitory control mechanisms are engaged to suppress the distraction they cause. This form of forgetting is considered to be adaptive because it reduces future interference. The effect of this proposed inhibition process on competing memories has, however, never been observed, as behavioral methods are 'blind' to retrieval dynamics and neuroimaging methods have not isolated retrieval of individual memories. We developed a canonical template tracking method to quantify the activation state of individual target memories and competitors during retrieval. This method revealed that repeatedly retrieving target memories suppressed cortical patterns unique to competitors. Pattern suppression was related to engagement of prefrontal regions that have been implicated in resolving retrieval competition and, critically, predicted later forgetting. Thus, our findings demonstrate a cortical pattern suppression mechanism through which remembering adaptively shapes which aspects of our past remain accessible.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
15 August 2018
In the published version of this article, a detail is missing from the Methods section "Experimental procedure." The following sentence is to be inserted at the end of its fourth paragraph: "If participants failed to respond within 3.5 s, we assumed that they were unable to successfully recognize the item and coded the corresponding trial as an error." The critical behavioral forgetting effect is significant irrespective of whether these timeouts are coded as errors (t23 = 4.91, P < 0.001) or as missing data (t23 = 3.31, P < 0.01). The original article has not been corrected.
References
Anderson, M. Rethinking interference theory: Executive control and the mechanisms of forgetting. J. Mem. Lang. 49, 415–445 (2003).
Hardt, O., Einarsson, E.O. & Nader, K. A bridge over troubled water: reconsolidation as a link between cognitive and neuroscientific memory research traditions. Annu. Rev. Psychol. 61, 141–167 (2010).
Anderson, M.C., Bjork, R.A. & Bjork, E.L. Remembering can cause forgetting: retrieval dynamics in long-term memory. J. Exp. Psychol. Learn. Mem. Cogn. 20, 1063–1087 (1994).
Storm, B.C. & Levy, B.J. A progress report on the inhibitory account of retrieval-induced forgetting. Mem. Cognit. 40, 827–843 (2012).
Kuhl, B.A., Dudukovic, N.M., Kahn, I. & Wagner, A.D. Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nat. Neurosci. 10, 908–914 (2007).
Wimber, M. et al. Neural markers of inhibition in human memory retrieval. J. Neurosci. 28, 13419–13427 (2008).
Kuhl, B.A., Bainbridge, W.A. & Chun, M.M. Neural reactivation reveals mechanisms for updating memory. J. Neurosci. 32, 3453–3461 (2012).
Kuhl, B.A., Rissman, J., Chun, M.M. & Wagner, A.D. Fidelity of neural reactivation reveals competition between memories. Proc. Natl. Acad. Sci. USA 108, 5903–5908 (2011).
Badre, D. & Wagner, A.D. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 45, 2883–2901 (2007).
Wimber, M., Rutschmann, R.M., Greenlee, M.W. & Bäuml, K.-H. Retrieval from episodic memory: neural mechanisms of interference resolution. J. Cogn. Neurosci. 21, 538–549 (2009).
Wimber, M. et al. Prefrontal dopamine and the dynamic control of human long-term memory. Transl. Psychiatry 1, e15 (2011).
Polyn, S.M., Natu, V.S., Cohen, J.D. & Norman, K.A. Category-specific cortical activity precedes retrieval during memory search. Science 310, 1963–1966 (2005).
Ritchey, M., Wing, E.A., LaBar, K.S. & Cabeza, R. Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cereb. Cortex 23, 2818–2828 (2013).
Staresina, B.P., Henson, R.N.A., Kriegeskorte, N. & Alink, A. Episodic reinstatement in the medial temporal lobe. J. Neurosci. 32, 18150–18156 (2012).
Chadwick, M.J., Hassabis, D., Weiskopf, N. & Maguire, E.A. Decoding individual episodic memory traces in the human hippocampus. Curr. Biol. 20, 544–547 (2010).
Poppenk, J. & Norman, K.A. Briefly cuing memories leads to suppression of their neural representations. J. Neurosci. 34, 8010–8020 (2014).
Anderson, M.C., Green, C. & McCulloch, K.C. Similarity and inhibition in long-term memory: evidence for a two-factor theory. J. Exp. Psychol. Learn. Mem. Cogn. 26, 1141–1159 (2000).
Spitzer, B. Finding retrieval-induced forgetting in recognition tests: a case for baseline memory strength. Front. Psychol. 5, 1102 (2014).
Kim, G., Lewis-Peacock, J.A., Norman, K.A. & Turk-Browne, N.B. Pruning of memories by context-based prediction error. Proc. Natl. Acad. Sci. USA 111, 8997–9002 (2014).
Detre, G.J., Natarajan, A., Gershman, S.J. & Norman, K.A. Moderate levels of activation lead to forgetting in the think/no-think paradigm. Neuropsychologia 51, 2371–2388 (2013).
Norman, K.A., Newman, E., Detre, G. & Polyn, S. How inhibitory oscillations can train neural networks and punish competitors. Neural Comput. 18, 1577–1610 (2006).
Alvarez, P. & Squire, L.R. Memory consolidation and the medial temporal lobe: a simple network model. Proc. Natl. Acad. Sci. USA 91, 7041–7045 (1994).
Norman, K.A. & O'Reilly, R.C. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol. Rev. 110, 611–646 (2003).
Hardt, O., Nader, K. & Nadel, L. Decay happens: the role of active forgetting in memory. Trends Cogn. Sci. 17, 111–120 (2013).
Chun, M.M. & Johnson, M.K. Memory: enduring traces of perceptual and reflective attention. Neuron 72, 520–535 (2011).
Desimone, R. & Duncan, J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222 (1995).
Gazzaley, A. & Nobre, A.C. Top-down modulation: bridging selective attention and working memory. Trends Cogn. Sci. 16, 129–135 (2012).
Anderson, M.C. & Spellman, B.A. On the status of inhibitory mechanisms in cognition: memory retrieval as a model case. Psychol. Rev. 102, 68–100 (1995).
Kastner, S. & Ungerleider, L.G. The neural basis of biased competition in human visual cortex. Neuropsychologia 39, 1263–1276 (2001).
Suzuki, M. & Gottlieb, J. Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat. Neurosci. 16, 98–104 (2013).
Gazzaley, A., Cooney, J.W., McEvoy, K., Knight, R.T. & D'Esposito, M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J. Cogn. Neurosci. 17, 507–517 (2005).
Cerf, M. et al. On-line, voluntary control of human temporal lobe neurons. Nature 467, 1104–1108 (2010).
Seidl, K.N., Peelen, M.V. & Kastner, S. Neural evidence for distracter suppression during visual search in real-world scenes. J. Neurosci. 32, 11812–11819 (2012).
Zanto, T.P., Rubens, M.T., Thangavel, A. & Gazzaley, A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat. Neurosci. 14, 656–661 (2011).
Squire, R.F., Noudoost, B., Schafer, R.J. & Moore, T. Prefrontal contributions to visual selective attention. Annu. Rev. Neurosci. 36, 451–466 (2013).
Nader, K. & Hardt, O. A single standard for memory: the case for reconsolidation. Nat. Rev. Neurosci. 10, 224–234 (2009).
Brady, T.F., Konkle, T., Alvarez, G.A. & Oliva, A. Visual long-term memory has a massive storage capacity for object details. Proc. Natl. Acad. Sci. USA 105, 14325–14329 (2008).
Rouder, J.N., Speckman, P.L., Sun, D., Morey, R.D. & Iverson, G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon. Bull. Rev. 16, 225–237 (2009).
Wager, T.D. & Nichols, T.E. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage 18, 293–309 (2003).
Macey, P.M., Macey, K.E., Kumar, R. & Harper, R.M. A method for removal of global effects from fMRI time series. Neuroimage 22, 360–366 (2004).
Kriegeskorte, N. Pattern-information analysis: from stimulus decoding to computational-model testing. Neuroimage 56, 411–421 (2011).
Kriegeskorte, N. & Kievit, R.A. Representational geometry: integrating cognition, computation, and the brain. Trends Cogn. Sci. 17, 401–412 (2013).
Kriegeskorte, N., Mur, M. & Bandettini, P. Representational similarity analysis - connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2, 4 (2008).
Misaki, M., Kim, Y., Bandettini, P.A. & Kriegeskorte, N. Comparison of multivariate classifiers and response normalizations for pattern-information fMRI. Neuroimage 53, 103–118 (2010).
Leys, C., Ley, C., Klein, O., Bernard, P. & Licata, L. Detecting outliers: do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 49, 764–766 (2013).
Acknowledgements
We thank B. Staresina and S. Hanslmayr for commenting on previous versions of the manuscript. This work was supported by a fellowship from the German Research Foundation (WI-3784/1-1) awarded to M.W. and by UK Medical Research Council grant MC-A060-5PR00 awarded to M.C.A.
Author information
Authors and Affiliations
Contributions
M.W. and M.C.A. designed the experiment, with important contributions by I.C. and N.K. M.W. conducted the experiment. M.W., A.A. and I.C. analyzed the data. All authors contributed to the analysis approach and to data interpretation. M.W. and M.C.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Behavioral results from the final recognition test split into the three picture categories.
Results demonstrate that forgetting on the final visual recognition task was not driven by one particular picture category. The difference in memory performance between competitors and their respective baseline items was significant in faces (t23 = 2.67; P = 0.007) and scenes (t23 = 2.35; P =.014), with a trend towards competitor suppression in objects (t23 = 0.85; P = 0.202). The lower panel shows that no category revealed significant target enhancement. Bars represent mean +/− s.e.m. across subjects.
Supplementary Figure 2 Results from the item-specific analysis when taking only correct trials into account.
Results are shown for (a) ventral visual cortex, and (b) the hippocampus. The second row shows the raw average correlation between recall activity and the templates of the current target (black), the templates of the current competitor (red), and the templates of their corresponding baseline items matched for category (light grey and pink, respectively). The middle row shows the mean difference between competitor-related similarity and similarity with the respective baseline templates (red solid), and the corresponding best linear fits (red dotted). Replicating the main results, evidence for item-specific competitor activation showed a significant (negative) linear trend across the four repetitions in ventral visual cortex (F1,23 = 12.47, P = 0.002), but not the hippocampus (F1,23 = 2.47, P = 0.130). Robust below-baseline suppression at the fourth repetition was present in both regions of interest (ventral visual cortex: t23 = 2.35, P = 0.014; hippocampus: t23 = 2.37, P = 0.013). The lower row shows the difference between target-related similarity and similarity with the respective baseline templates. Relative to baseline templates from the same category, similarity with the target template showed a significant (positive) linear trend in the ventral visual cortex (F1,23 = 4.62, P = 0.042), but not the hippocampus (F1,23 = 2.61, P = 0.120), replicating the results including all trials. On the final (fourth) recall attempt, similarity with the target templates was significantly higher than similarity with the category-matched baseline items in the hippocampus (t23 = 2.42, P = 0.012), but not in ventral visual cortex (t23 = 0.13, P = 0.449). All line plots represent means +/− s.e.m. across subjects.
Supplementary Figure 3 Slope of competitor suppression as a function of voxel diagnosticity.
The slope (best fitting ML estimate) of mean signal down-regulation across the four recall repetitions is shown separately for voxels diagnostic for the target item (black) and competitor (red). Along the x-axis, diagnosticity of the voxels (as extracted from the weights of our item-specific linear pattern classifiers) increases by 10%, starting at 50% (as lower bins would indicate presence of the control items that were used as baselines in our binary item-specific classifiers). Bin 1 contains the most diagnostic voxels, bin 5 the least diagnostic voxels. The same data are plotted twice for illustrative purposes, on the left by plotting each bin separately, and on the right by cumulating the slope values across bins, starting with the highest diagnosticity bin. In both cases, only the bin with the 10% most diagnostic voxels showed a significantly negative suppression slope (see statistics in the main text; * P < 0.05).
Supplementary Figure 4 Categorical classifier results dependent on button presses during selective retrieval.
Accuracy of categorical linear classifiers (SVMs) in predicting the target and competitor categories (always relative to the currently irrelevant, non-involved category) from multivoxel patterns in ventral visual cortex, depending on the button press responses subjects gave during the selective recall task. Separate line plots show mean accuracy on trials on which subjects indicated recalling the target category (upper), recalling the competitor category (middle), or being unable to retrieve the correct association (lower). Asterisks indicate classification performance significantly (P < 0.05) higher than chance. Lines represent mean classification performance (error bars show s.e.m. across subjects).
Supplementary Figure 5 Control analyses indicating that the templates were not measurably affected by condition.
Results are shown for (a) ventral visual cortex, and (b) hippocampus. The templates of targets and competitors did not differ from the corresponding baseline templates in signal-to-noise ratio (mean/standard deviation), in Shannon’s entropy, or in the average correlation between templates from the same item type (see Supplementary Table 2 for exact t-values, p-values and Bayes factors).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1 and 2 (PDF 918 kb)
Rights and permissions
About this article
Cite this article
Wimber, M., Alink, A., Charest, I. et al. Retrieval induces adaptive forgetting of competing memories via cortical pattern suppression. Nat Neurosci 18, 582–589 (2015). https://doi.org/10.1038/nn.3973
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3973
This article is cited by
-
Cognitive perspectives on maintaining physicians’ medical expertise: II. Acquiring, maintaining, and updating cognitive skills
Cognitive Research: Principles and Implications (2023)
-
Behavioral representational similarity analysis reveals how episodic learning is influenced by and reshapes semantic memory
Nature Communications (2023)
-
The critical importance of timing of retrieval practice for the fate of nonretrieved memories
Scientific Reports (2023)
-
Acute exercise on memory: application of the retrieval-induced forgetting paradigm
Psychological Research (2023)
-
Dynamic targeting enables domain-general inhibitory control over action and thought by the prefrontal cortex
Nature Communications (2022)