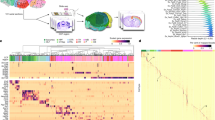
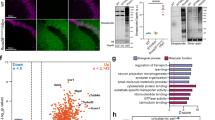
Abstract
Brain transcriptome and connectome maps are being generated, but an equivalent effort on the proteome is currently lacking. We performed high-resolution mass spectrometry–based proteomics for in-depth analysis of the mouse brain and its major brain regions and cell types. Comparisons of the 12,934 identified proteins in oligodendrocytes, astrocytes, microglia and cortical neurons with deep sequencing data of the transcriptome indicated deep coverage of the proteome. Cell type–specific proteins defined as tenfold more abundant than average expression represented about a tenth of the proteome, with an overrepresentation of cell surface proteins. To demonstrate the utility of our resource, we focused on this class of proteins and identified Lsamp, an adhesion molecule of the IgLON family, as a negative regulator of myelination. Our findings provide a framework for a system-level understanding of cell-type diversity in the CNS and serves as a rich resource for analyses of brain development and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lein, E.S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Thompson, C.L. et al. A high-resolution spatiotemporal atlas of gene expression of the developing mouse brain. Neuron 83, 309–323 (2014).
Cahoy, J.D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008).
Doyle, J.P. et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell 135, 749–762 (2008).
Heiman, M. et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell 135, 738–748 (2008).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Kang, H.J. et al. Spatio-temporal transcriptome of the human brain. Nature 478, 483–489 (2011).
Johnson, M.B. et al. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron 62, 494–509 (2009).
Hickman, S.E. et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16, 1896–1905 (2013).
Butovsky, O. et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 (2014).
Vogel, C. & Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 13, 227–232 (2012).
Kitchen, R.R., Rozowsky, J.S., Gerstein, M.B. & Nairn, A.C. Decoding neuroproteomics: integrating the genome, translatome and functional anatomy. Nat. Neurosci. 17, 1491–1499 (2014).
Beck, M., Claassen, M. & Aebersold, R. Comprehensive proteomics. Curr. Opin. Biotechnol. 22, 3–8 (2011).
Mann, M., Kulak, N.A., Nagaraj, N. & Cox, J. The coming age of complete, accurate, and ubiquitous proteomes. Mol. Cell 49, 583–590 (2013).
Nagaraj, N. et al. Deep proteome and transcriptome mapping of a human cancer cell line. Mol. Syst. Biol. 7, 548 (2011).
Wilhelm, M. et al. Mass-spectrometry-based draft of the human proteome. Nature 509, 582–587 (2014).
Kim, M.S. et al. A draft map of the human proteome. Nature 509, 575–581 (2014).
Geiger, T., Wehner, A., Schaab, C., Cox, J. & Mann, M. Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Mol. Cell Proteomics 11, M111.014050 (2012).
Lundberg, E. et al. Defining the transcriptome and proteome in three functionally different human cell lines. Mol. Syst. Biol. 6, 450 (2010).
Pontén, F. et al. A global view of protein expression in human cells, tissues, and organs. Mol. Syst. Biol. 5, 337 (2009).
Barres, B.A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440 (2008).
Sherman, D.L. & Brophy, P.J. Mechanisms of axon ensheathment and myelin growth. Nat. Rev. Neurosci. 6, 683–690 (2005).
Aggarwal, S., Yurlova, L. & Simons, M. Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol. 21, 585–593 (2011).
Molofsky, A.V. et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 26, 891–907 (2012).
Hanisch, U.K. & Kettenmann, H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394 (2007).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Nagaraj, N. et al. System-wide perturbation analysis with nearly complete coverage of the yeast proteome by single-shot ultra HPLC runs on a bench top Orbitrap. Mol. Cell Proteomics 11, M111.013722 (2012).
Hebenstreit, D. et al. RNA sequencing reveals two major classes of gene expression levels in metazoan cells. Mol. Syst. Biol. 7, 497 (2011).
Beck, M. et al. The quantitative proteome of a human cell line. Mol. Syst. Biol. 7, 549 (2011).
de Sousa Abreu, R., Penalva, L.O., Marcotte, E.M. & Vogel, C. Global signatures of protein and mRNA expression levels. Mol. Biosyst. 5, 1512–1526 (2009).
Maier, T., Guell, M. & Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 583, 3966–3973 (2009).
Cox, J. et al. MaxLFQ allows accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction. Mol. Cell. Proteomics 13, 2513–2526 (2014).
Wiśśniewski, J.R. et al. Extensive quantitative remodeling of the proteome between normal colon tissue and adenocarcinoma. Mol. Syst. Biol. 8, 611 (2012).
Marguerat, S. et al. Quantitative analysis of fission yeast transcriptomes and proteomes in proliferating and quiescent cells. Cell 151, 671–683 (2012).
Snead, M.P. & Yates, J.R. Clinical and molecular genetics of Stickler syndrome. J. Med. Genet. 36, 353–359 (1999).
Charles, P. et al. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc. Natl. Acad. Sci. USA 97, 7585–7590 (2000).
Pimenta, A.F. et al. The limbic system-associated membrane protein is an Ig superfamily member that mediates selective neuronal growth and axon targeting. Neuron 15, 287–297 (1995).
Schofield, P.R. et al. Molecular characterization of a new immunoglobulin superfamily protein with potential roles in opioid binding and cell contact. EMBO J. 8, 489–495 (1989).
Struyk, A.F. et al. Cloning of neurotrimin defines a new subfamily of differentially expressed neural cell adhesion molecules. J. Neurosci. 15, 2141–2156 (1995).
Reed, J., McNamee, C., Rackstraw, S., Jenkins, J. & Moss, D. Diglons are heterodimeric proteins composed of IgLON subunits, and Diglon-CO inhibits neurite outgrowth from cerebellar granule cells. J. Cell Sci. 117, 3961–3973 (2004).
Gil, O.D., Zanazzi, G., Struyk, A.F. & Salzer, J.L. Neurotrimin mediates bifunctional effects on neurite outgrowth via homophilic and heterophilic interactions. J. Neurosci. 18, 9312–9325 (1998).
Spiegel, I. et al. A central role for Necl4 (SynCAM4) in Schwann cell-axon interaction and myelination. Nat. Neurosci. 10, 861–869 (2007).
Maurel, P. et al. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J. Cell Biol. 178, 861–874 (2007).
Reed, J.E. et al. Expression of cellular adhesion molecule 'OPCML' is down-regulated in gliomas and other brain tumours. Neuropathol. Appl. Neurobiol. 33, 77–85 (2007).
Moghaddas Gholami, A. et al. Global proteome analysis of the NCI-60 cell line panel. Cell Rep. 4, 609–620 (2013).
Azimifar, S.B., Nagaraj, N., Cox, J. & Mann, M. Cell type–resolved quantitative proteomics of murine liver. Cell Metab. 20, 1076–1087 (2014).
Bayés, A. et al. Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nat. Neurosci. 14, 19–21 (2011).
Gil, O.D. et al. Complementary expression and heterophilic interactions between IgLON family members neurotrimin and LAMP. J. Neurobiol. 51, 190–204 (2002).
Fields, R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 31, 361–370 (2008).
Innos, J., Koido, K., Philips, M.A. & Vasar, E. Limbic system associated membrane protein as a potential target for neuropsychiatric disorders. Front. Pharmacol 4, 32 (2013).
Trajkovic, K. et al. Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. J. Cell Biol. 172, 937–948 (2006).
Fitzner, D. et al. Myelin basic protein-dependent plasma membrane reorganization in the formation of myelin. EMBO J. 25, 5037–5048 (2006).
Regen, T. et al. CD14 and TRIF govern distinct responsiveness and responses in mouse microglial TLR4 challenges by structural variants of LPS. Brain Behav. Immun. 25, 957–970 (2011).
Shevchenko, A., Tomas, H., Havlis, J., Olsen, J.V. & Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 (2006).
Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896–1906 (2007).
Wiśniewski, J.R., Zougman, A., Nagaraj, N. & Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362 (2009).
Cox, J. et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 (2011).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Lawrence, M. et al. Software for computing and annotating genomic ranges. PLOS Comput. Biol. 9, e1003118 (2013).
Schnell, C. et al. The multispecific thyroid hormone transporter OATP1C1 mediates cell-specific sulforhodamine 101-labeling of hippocampal astrocytes. Brain Struct. Funct. 220, 193–203 (2015).
Snaidero, N. et al. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156, 277–290 (2014).
Acknowledgements
We would like to acknowledge the PRIDE Team for upload of proteomics raw data. We thank J. Cox, M.Y. Hein and K. Mayr for helpful discussions, and C. Velte, N. Schwedhelm-Dornmeyer, S. Safaiyan, G. Sowa and M. Dodel for technical assistance. Illumina sequencing was performed by R. Reinhardt at the Max-Planck Genome Center Cologne. The work was supported grants from the German Research Foundation (SI 746/9-1; 10-1; TRR43; RO 4076/3-1), the Tschira-Stiftung, and grants from the Estonian Research Council (IUT20-41 and PUT129). The research leading to these results has received funding from the European Commission under FP7 GA n°ERC-2012-SyG_318987 – ToPAG and MC-ITN IN-SENS (#607616). S.S. received a PhD scholarship from the Boeringer-Ingelheim Fonds and N.K. this recipient of a Marie-Curie fellowship from the INSENS/ FP7-PEOPLE-2013 (607616) framework.
Author information
Authors and Affiliations
Contributions
K.S., M.M. and M.S. designed the experiments. K.S., S.S., C.G.B., N.K., N.M.-H., L.C. and U.-K.H. performed the experiments. K.S., S.S., S.T., N.K., M.J.R., M.M. and M.S. analyzed the data. K.K. and M.-A.P. provided materials. K.S., M.M. and M.S. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 (PDF 4107 kb)
Supplementary Table 1
Protein expression data of adult mouse brain and cultured CNS cell types measured as fractionated samples. (XLSX 7700 kb)
Supplementary Table 2
Protein expression data of mouse brain regions (P60), acutely isolated CNS cell types and cerebellum development (P5, P14, P24) measured by 'single-run' analysis. (XLSX 5694 kb)
Supplementary Table 3
RNA-Seq expression data for the cultured CNS cell types. (XLSX 4139 kb)
Supplementary Table 4
Gene ontology enrichment analysis of the genes expressed exclusively at the transcript level and lack evidence of expression at the protein level. (XLSX 15 kb)
Supplementary Table 5
Protein expression data of cultured CNS cell types for individual replicates and developmental stage. (XLSX 2930 kb)
Supplementary Table 6
Differentially expressed proteins in cultured CNS cell types. (XLSX 2671 kb)
Supplementary Table 7
Differentially expressed proteins in cortical neurons and cerebellar granule neurons. (XLSX 8287 kb)
Supplementary Table 8
Clusters based enrichment analysis for the cultured CNS cell types. (XLSX 68 kb)
Supplementary Table 9
Cluster based enrichment analysis for cerebellar granule neurons. (XLSX 20 kb)
Supplementary Table 10
Comparison of annotation terms (KEGG pathway, GO terms and Corum) between the cultured CNS cell types resolved to individual replicates and developmental stage. (XLSX 375 kb)
Supplementary Table 11
GOCC enrichment of proteins >10 fold enriched in cultured CNS cell types. (XLSX 179 kb)
Supplementary Table 12
GOCC enrichment of transcripts >10 fold enriched in cultured CNS cell types. (XLSX 21 kb)
Supplementary Table 13
Differentially expressed proteins in isolated CNS cell types. (XLSX 3391 kb)
Supplementary Table 14
Comparison of annotation terms (GO terms and Corum) between the cultured and isolated CNS cell types resolved to individual replicates. (XLSX 682 kb)
Supplementary Table 15
A list of the most abundant brain-enriched (>10 fold enrichment as compared to the liver) proteins. (XLSX 51 kb)
Supplementary Table 16
Comparison of annotation terms (KEGG pathways and GO terms) between the mouse brain and liver. (XLSX 100 kb)
Supplementary Table 17
Protein expression in mouse brain regions. (XLSX 5399 kb)
Supplementary Table 18
Differentially expressed 2,901 proteins in mouse brain regions. (XLSX 2152 kb)
Rights and permissions
About this article
Cite this article
Sharma, K., Schmitt, S., Bergner, C. et al. Cell type– and brain region–resolved mouse brain proteome. Nat Neurosci 18, 1819–1831 (2015). https://doi.org/10.1038/nn.4160
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4160
This article is cited by
-
Quantitative proteomics of cerebrospinal fluid from African Americans and Caucasians reveals shared and divergent changes in Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
The Alzheimer’s disease-linked protease BACE1 modulates neuronal IL-6 signaling through shedding of the receptor gp130
Molecular Neurodegeneration (2023)
-
Translational profiling identifies sex-specific metabolic and epigenetic reprogramming of cortical microglia/macrophages in APPPS1-21 mice with an antibiotic-perturbed-microbiome
Molecular Neurodegeneration (2023)
-
Axonal energy metabolism, and the effects in aging and neurodegenerative diseases
Molecular Neurodegeneration (2023)
-
Microglial cannabinoid receptor type 1 mediates social memory deficits in mice produced by adolescent THC exposure and 16p11.2 duplication
Nature Communications (2023)