Abstract
Memories, similar to the internal representation of space, can be recalled at different resolutions ranging from detailed events to more comprehensive, multi-event narratives. Single-cell recordings in rodents have suggested that different spatial scales are represented as a gradient along the hippocampal axis. We found that a similar organization holds for human episodic memory: memory representations systematically vary in scale along the hippocampal long axis, which may enable the formation of mnemonic hierarchies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Eichenbaum, H., Dudchenko, P., Wood, E., Shapiro, M. & Tanila, H. Neuron 23, 209–226 (1999).
Fanselow, M.S. & Dong, H. Neuron 65, 7–19 (2010).
Ritchey, M., Montchal, M., Yonelinas, A. & Ranganath, C. Elife 4, 1–19 (2015).
Liang, J.C., Wagner, A.D. & Preston, A.R. Cereb. Cortex 23, 80–96 (2013).
Strange, B.A., Witter, M.P., Lein, E.S. & Moser, E.I. Nat. Rev. Neurosci. 15, 655–669 (2014).
Horner, A.J. & Burgess, N. Curr. Biol. 24, 988–992 (2014).
McKenzie, S. et al. Neuron 83, 202–215 (2014).
Poppenk, J., Evensmoen, H.R., Moscovitch, M. & Nadel, L. Trends Cogn. Sci. 17, 230–240 (2013).
Milivojevic, B. & Doeller, C.F. J. Exp. Psychol. 142, 1231–1241 (2013).
Milivojevic, B., Vicente-Grabovetsky, A. & Doeller, C.F. Curr. Biol. 25, 821–830 (2015).
Shohamy, D. & Wagner, A. Neuron 60, 378–389 (2008).
Zeithamova, D., Dominick, A.L. & Preston, A.R. Neuron 75, 168–179 (2012).
Staresina, B.P. & Davachi, L. Neuron 63, 267–276 (2009).
O'Keefe, J. & Nadel, L. The Hippocampus as a Cognitive Map (Oxford University Press, 1978).
Komorowski, R.W. et al. J. Neurosci. 33, 8079–8087 (2013).
Komorowski, R.W., Manns, J.R. & Eichenbaum, H. J. Neurosci. 29, 9918–9929 (2009).
Kumaran, D., Summerfield, J.J., Hassabis, D. & Maguire, E. Neuron 63, 889–901 (2009).
van Kesteren, M.T., Fernández, G., Norris, D.G. & Hermans, E.J. Proc. Natl. Acad. Sci. USA 107, 7550–7555 (2010).
Staresina, B.P., Fell, J., Do Lam, A.T.A., Axmacher, N. & Henson, R.N. Nat. Neurosci. 15, 1167–1173 (2012).
Schapiro, A.C., Rogers, T.T., Cordova, N.I., Turk-Browne, N.B. & Botvinick, M.M. Nat. Neurosci. 16, 486–492 (2013).
Poser, B.A., Koopmans, P., Witzel, T., Wald, L. & Barth, M. Neuroimage 51, 261–266 (2010).
Cusack, R. et al. Front. Neuroinform. 8, 90 (2015).
Coupé, P., Yger, P., Prima, S., Hellier, P. & Kervrann, C. IEEE Trans. Med. Imaging 27, 425–441 (2007).
Andersson, J.L., Hutton, C., Ashburner, J., Turner, R. & Friston, K. Neuroimage 13, 903–919 (2001).
Glover, G.H., Li, T.Q. & Ress, D. Magn. Reson. Med. 44, 162–167 (2000).
Ashburner, J. & Friston, K.J. Neuroimage 26, 839–851 (2005).
Mumford, J.A., Turner, B.O., Ashby, F.G. & Poldrack, R. Neuroimage 59, 2636–2643 (2012).
Kriegeskorte, N. & Kievit, R. Trends Cogn. Sci. 17, 401–412 (2013).
Maldjian, J.A., Laurienti, P.J., Kraft, R. & Burdette, J.H. Neuroimage 19, 1233–1239 (2003).
Acknowledgements
The authors would like to thank A. Backus, S. Bosch and L. Deuker for useful discussions, N. Müller for help during stimulus development, and K. Georgelou for help during behavioral pilot work. This work was supported by the European Research Council (ERC-StG RECONTEXT 261177) and the Netherlands Organization for Scientific Research (NWO-Vidi 452-12-009).
Author information
Authors and Affiliations
Contributions
S.H.P.C. designed the experiment, collected the data, analyzed the data and wrote the manuscript. B.M. designed the experiment, supervised data analysis and wrote the manuscript. C.F.D. conceived and designed the experiment, supervised the project, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
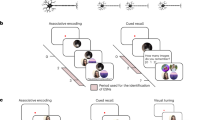
Supplementary Figure 1 Schematic overview of task design and main analysis logic.
(a) In each scanning run, one narrative was presented. Each run contained 5 phases (Phase 1, Link-phase 1, Phase 2, Link-phase 2, and Phase 3). (b) In Phases 1, 2 and 3, four events (A, B, C, X) were presented 6 times each in a pseudorandom order (an event was never presented twice in a row, and all four events were presented before they were repeated). In the link-phases, a link event was presented 6 times in a row. (c) When computing the similarity matrix, only neural similarity between events within the same phase were considered.
Supplementary Figure 2 Overview of baseline corrections in the fMRI analyses.
(a) Schematic illustration of predicted configurations of mnemonic networks following narrative-link presentations. Our main conclusions concern the formation of mnemonic networks that contain only those events that were part of the narrative, while other events that were irrelevant to the narrative were not included into these mnemonic networks. A, B and C were narrative-events, X was the control event, L1 and L2 were link-events 1 and 2. (b) Bars show neural similarity for Phase 1 (P1), Phase 2 (P2) and Phase 3 (P3) of the experiment, plotted separately for AX, BX and CX pairs and the different ROIs, respectively. As expected, similarity measures did not differ between event pairs AX, BX and CX. A repeated-measures ANOVA with Hemisphere (L, R), Event-pair (AX, BX, CX), ROI (anterior, mid-portion, posterior), and Phase (1, 2, 3) as within-subject factors showed that there was no significant main effect of ROI (F2,56 = 0.534, p = 0.589), Hemisphere (F1,28 = 0.698, p = 0.411) or Event-pair (F2,56 = 0.217, p = 0.805), nor were there any significant interaction effects between the factors (all p > 0.15). Control analysis BX baseline similarity: To determine whether changes in baseline BX similarity were driving the effects of the main analysis reported in Figure 2, we also re-ran the analysis without taking the baseline into account, and found similar results: the mnemonic-scaling contrast in the repeated-measures ANOVA with Narrative scale (small, medium, large), ROI (anterior, mid-portion, posterior), and Hemisphere (L, R) as within-subject factors, showed a significant interaction effect (F1,28 = 5.282, p < 0.05). Additionally, analysing this baseline enabled us to rule out whether the increasing amount of information and time throughout the task could have explained the mnemonic scaling effect. Thus, we examined BX using a repeated-measures ANOVA with ROI (anterior, mid-portion, posterior), Hemisphere (L, R), and Phase (1, 2, 3) as within-subject factors. There was no significant ROI by phase interaction (F4,112 = 1.147, p = 0.338) and also no significant main effect of ROI (F2,56 = 0.376, p = 0.689).
Supplementary Figure 3 Increasing memory scale along the hippocampal long axis, separately for each hemisphere.
Model evidence (parameter estimates) separately for left and right hippocampus, and separately for the three ROIs and three network scales (mean ± S.E.M., N = 29). There was no significant difference between left and right hippocampus (F1,28 = 0.073, p = 0.789).
Supplementary Figure 4 Narrative=scaling effects along the hippocampal long axis are not due to mean BOLD-signal fluctuations.
We included three extra nuisance regressors in the first-level model for each run of each participant. These three extra nuisance regressors accounted for the mean univariate signal amplitude averaged across all voxels of anterior (Regressor 1), mid-portion (Regressor 2), and posterior (Regressor 3) hippocampus for each volume in the task. Then, based on these first-level models we ran the same analysis as reported in Figure 2. Bars show model evidence (parameter estimates) averaged across left and right hippocampus (mean ± S.E.M.) separately for the three ROIs and three scales (N=29). The specific interaction effect that we predicted in a reduced a priori model (i.e. small-scale network in posterior portion, medium-scale network in mid-portion, large-scale network in anterior portion) with Narrative scale (small, medium, large), ROI (posterior, mid-portion, anterior), and Hemisphere (L, R) as within-subject factors, remained significant (F1,28 = 15.1, p = 0.001). The full repeated-measures ANOVA with Narrative scale (small, medium, large), ROI (posterior, mid-portion, anterior), and Hemisphere (L, R) as within-subject factors also showed a significant full interaction effect (Narrative-scale by ROI interaction: F4,112 = 2.725, p = 0.033). Based on these results we conclude that the fluctuation of the univariate signal between phases and/or between ROIs did not explain the reported results.
Supplementary Figure 5 Effects of inferred associations.
Increase of A-C similarity relative to the control condition B-X, for full-integration (grey bars, N=12) and partial-integration (orange bars, N=16) performance groups separately. Predicted A-C increase is the crucial difference between the medium-scale network and the large-scale network. When looking at this A-C association in isolation, it becomes clear that A-C increase is only present in the anterior portion of the hippocampus (main effect over both groups, anterior portion: F1,28 = 8.85, p < 0.01), but not in the mid-portion or posterior portion of the hippocampus (mid-portion: F1,28 = 0.003, p = 0.96; posterior portion: F1,28 = 0.89, p = 0.35, respectively). For 1 participant these behavioural data were missing. The main fMRI results were not affected by inclusion or exclusion of this participant.
Supplementary Figure 6 Strength of the memory-scale effect along the hippocampal long axis is associated with narrative integration.
(a) Results of the recall task. One group of participants integrated all five events from each of the four narratives into four unified narratives (N = 12, the ‘full-integration’ group). The rest of the group (N = 16, the ‘partial-integration’ group) exhibited lower levels of narrative integration. For 1 participant these behavioural data were missing. Main fMRI results were not affected by the inclusion or exclusion of this participant. (b) Partial-integration group (N = 16), scale-by-ROI: F4,60 = 1.07, p = 0.38. (c) Full integration group (N = 12), scale-by-ROI: F4,44 = 3.97, p < 0.01. (b/c): Model evidence (parameter estimates) is plotted for each of the three ROIs, three scales, and two groups, averaged across hemispheres (mean ± S.E.M.); *p <.05, **p <.01. Note that the ‘full-integration’ group had fewer participants than the ‘partial-integration’ group, and the lack of significant interaction in the ‘partial-integration’ group is unlikely to reflect reduced power.
Supplementary Figure 7 Strength of the memory-scale effect along the hippocampal long axis is associated with narrative integration.
Neural similarity for each pairwise comparison (A-B, B-C, A-C, and B-X) in each phase (1, 2, 3) in each ROI (from left to right: posterior portion, mid-portion, and anterior portion of the hippocampus) averaged over the hemispheres for full-integration (grey bars, N = 12) and partial-integration (orange bars, N=16) groups separately. AB = yellow, BC = green, AC = blue, and BX = red. The increasing memory scale along the long axis was strongest in the full-integration group. 1: A-B neural similarity increased after L1 in the full-integration group only. 2: B-C neural similarity increased after L2 in the full-integration group only. 3: A-B neural similarity decreased between Phase 2 and Phase 3 for the full-integration group, suggesting a dynamic shift to B-C, however, the partial-integration participants showed increase in A-B between Phase 2 and Phase 3. Together, these results indicate that the full-integration group showed a dynamic activity pattern in the posterior portion of the hippocampus, with fast linking between A-B immediately after L1, followed by a dynamic shift towards B-C after L2. The partial-integration group did not show this dynamic activity pattern. In fact, the data suggest that these participants were still linking A-B even though both L1 and L2 were already presented. This might be a reason why they failed to link B-C. 4: The A-C neural similarity increased between Phase 2 and 3 for both groups, in the anterior portion of the hippocampus only. 5: In the mid-portion and anterior portion of the hippocampus, the full-integration group showed only a decrease for B-X neural similarity between Phase 2 and 3. In contrast to the partial-integration group, who showed this decrease in neural similarity for B-X already between Phase 1 and 2 in mid-portion and anterior portion of the hippocampus. Thus, these data suggest that it might be beneficial for a successful narrative construction to keep all possible narrative-events ‘online’ until all necessary information (i.e. L1 and L2) is available.
Supplementary Figure 8 Schematic of our design in comparison with classical transitive-inference paradigms.
A, B, C are narrative-events, L1 and L2 are link-events 1 and 2. Both transitive-inference studies as well as our design require the participants to link A and C through B. In our design participant are requested to link A and B through L1, and B and C through L2. This means that the link between A and C is through L1, B and L2. Thus, the link between A and C in our design can be visualized by the top row, which is different from the transitive inference paradigm (bottom row). In our design, the large-scale network that includeed the link between A and C is represented in anterior hippocampus. However, previous studies12 find such effects also in the mid-portion of the hippocampus. Differences in the degree of event complexity between experimental designs may account for the difference in the results. Indeed there are similarities between the transitive-inference paradigms used by Preston and colleagues12 and our design. However, we feel there are considerable differences as well, which could explain this discrepancy in the results. The networks created by our design are more complex because a single event already consists of multiple items. Thus, our design requires participants to combine multiple complex events into a larger network. Additionally, transitive-inference studies require the participants to link A and C through B (bottom row). In contrast, in our design participants were requested to link A and B through L1, and B and C through L2 (top row). This means that the link between A and C is through L1, B and L2. Thus, the link between A and C in our design is different from the A-C link in the transitive-inference paradigm. Also, the order in which events are presented in our design is different from the inference experiments. In transitive inference paradigms, participants learn A-B and B-C associations at different points in time (i.e. in different trials). In our design, A, B and C are presented before and after the linking-events (first L1, and then L2). Thus, participants were required to remember the A, B and C events in order to link them by the separately presented link-events. This could potentially explain why our large-scale network is present in anterior hippocampus, while earlier study designs with an inferred AC link found their effects in mid-hippocampus12.
Supplementary Figure 9 Increasing memory scale along the hippocampal long axis, separately for both hemispheres.
Neural similarity for each pairwise comparison (A-B, B-C, A-C, and B-X) in each Phase (1, 2, 3) in each ROI (from left to right: posterior portion, mid-portion, and anterior portion of the hippocampus) for left (above) and right (below) hemispheres separately (N=29). AB = yellow, BC = green, AC = blue, and BX = red. Alternative accounts about functional specialisation of anterior and posterior hippocampus: Our results demonstrate that a mnemonic gradient underlies organisation of human episodic memories, which may relate to the gradient of the scale of encoded space. However, earlier studies have proposed other functional dissociations within the hippocampus. In short, other accounts about the differences between anterior and posterior hippocampal processing (as discussed in Poppenk et al., 2013) are: [1] Anterior (emotion/motivation) – posterior (other cognition); [2] Anterior (encoding) – posterior (retrieval); [3] Anterior (other cognition) – posterior (spatial memory); [4] Anterior (vestibular memory and navigation) – posterior (visual memory and navigation); [5] Anterior (global spatial representation) – posterior (local spatial representation); [6] Anterior (gist) – posterior (detail). Our data are in line with Account 5, which is largely based on the observation of an almost linear increase in the size of place fields along the long axis of the hippocampus in rodents. Our results suggest a differently scaled representation of episodic memories along the hippocampal long axis in humans. This suggests that there could be a general coding mechanism in the hippocampus responsible for spatial as well as episodic memories, and our results extend Account 5 into a non-spatial domain of memory. Our data also accord with the gist-detail dissociation proposed by Account 6. However, with the present design it was not possible to rigorously test differences in representation of gist versus detail. Accounts 1 and 3 both predict that specific sub-regions of the hippocampus are responsible for only emotion or spatial memory, respectively. Our results show involvement of the entire hippocampus, from anterior to posterior, in episodic memory and memory integration processes, which is difficult to reconcile with the hippocampal dissociation proposed by Accounts 1 and 3. Accounts 2 and 4 concern distinct memory functions, encoding vs. retrieval (Account 2) and vestibular vs. visual memory (Account 4), respectively, which were not directly testable with our experimental approach.
Supplementary Figure 10 Results of the behavioral control experiment.
It was important to determine how participants’ behavior was affected by the first link-event. However, in the main experiment we decided to conduct all behavioral tasks after the end of the MRI session to avoid possible interference with the narrative task. In order to draw conclusions about the interpretation of the initial link before the second link-event was presented, we performed a behavioral experiment with a separate group of participants (N = 21, 8 males, aged 18 to 37 years, mean age 24.2). In this experiment participants were presented with screenshots of events A, B and C and had to choose which event (A or C) belonged to event A. They received this question without seeing link-event 2. Participants performed at ceiling level for the initial-link associations directly after link-phase 1 (94% correct on average; for the 4 stories separately 95%, 98%, 90%, 93% correct).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Table 1 (PDF 1270 kb)
Rights and permissions
About this article
Cite this article
Collin, S., Milivojevic, B. & Doeller, C. Memory hierarchies map onto the hippocampal long axis in humans. Nat Neurosci 18, 1562–1564 (2015). https://doi.org/10.1038/nn.4138
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4138
This article is cited by
-
Non-invasive temporal interference electrical stimulation of the human hippocampus
Nature Neuroscience (2023)
-
Neural dynamics underlying associative learning in the dorsal and ventral hippocampus
Nature Neuroscience (2023)
-
The human brain reactivates context-specific past information at event boundaries of naturalistic experiences
Nature Neuroscience (2023)
-
Recruitment of a long-term memory supporting neural network during repeated maintenance of a multi-item abstract visual image in working memory
Scientific Reports (2022)
-
Predicting memory from the network structure of naturalistic events
Nature Communications (2022)