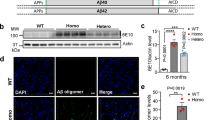
Abstract
Experimental studies of Alzheimer's disease have largely depended on transgenic mice overexpressing amyloid precursor protein (APP). These mice, however, suffer from artificial phenotypes because, in addition to amyloid β peptide (Aβ), they overproduce other APP fragments. We generated knock-in mice that harbor Swedish and Beyreuther/Iberian mutations with and without the Arctic mutation in the APP gene. The mice showed typical Aβ pathology, neuroinflammation and memory impairment in an age-dependent manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jonsson, T. et al. Nature 488, 96–99 (2012).
Hardy, J. & Allsop, D. Trends Pharmacol. Sci. 12, 383–388 (1991).
Hsiao, K. et al. Science 274, 99–102 (1996).
Sturchler-Pierrat, C. et al. Proc. Natl. Acad. Sci. USA 94, 13287–13292 (1997).
Citron, M. et al. Nature 360, 672–674 (1992).
Lichtenthaler, S.F. et al. Proc. Natl. Acad. Sci. USA 96, 3053–3058 (1999).
Guardia-Laguarta, C. et al. J. Neuropathol. Exp. Neurol. 69, 53–59 (2010).
Forman, M.S. et al. J. Biol. Chem. 272, 32247–32253 (1997).
Saido, T.C. et al. Neuron 14, 457–466 (1995).
Mitani, Y. et al. J. Neurosci. 32, 2037–2050 (2012).
Higuchi, M. et al. FASEB J. 26, 1204–1217 (2012).
Roberson, E.D. et al. Science 316, 750–754 (2007).
Liu, C.C., Kanekiyo, T., Xu, H. & Bu, G. Nat. Rev. Neurol. 9, 106–118 (2013).
Hashimoto, T. et al. J. Biol. Chem. 286, 27081–27091 (2011).
Tsubuki, S., Takaki, Y. & Saido, T.C. Lancet 361, 1957–1958 (2003).
Kalimo, H. et al. Acta Neuropathol. Commun. 1, 60 (2013).
Cheng, I.H. et al. Nat. Med. 10, 1190–1192 (2004).
Cash, D.M. et al. Neurology 81, 1425–1433 (2013).
Nilsson, P. et al. Cell Reports 5, 61–69 (2013).
Takano, J. et al. J. Biol. Chem. 280, 16175–16184 (2005).
Saito, T. et al. Nat. Med. 11, 434–439 (2005).
Iwata, N. et al. J. Neurosci. 24, 991–998 (2004).
Enya, M. et al. Am. J. Pathol. 154, 271–279 (1999).
Saito, T. et al. Nat. Neurosci. 14, 1023–1032 (2011).
Sarnyai, Z. et al. Proc. Natl. Acad. Sci. USA 97, 14731–14736 (2000).
Acknowledgements
We thank C.A. Lemere (Brigham and Woman's Hospital) and M. Higuchi (National Institute of Radiological Science) for valuable discussions. We also thank E. Takano, R. Fujioka and Y. Tomita for technical assistance. This work was supported by research grants from RIKEN Brain Science Institute, the Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Health and Welfare, grants-in-aid for the Molecular Imaging Program, Japan Science and Technology Agency Precursory Research for Embryonic Science and Technology, the Cell Science Research Foundation and the Takeda Science Foundation.
Author information
Authors and Affiliations
Contributions
This study was designed by T.S. and T.C.S. Generation of the knock-in mice was supported by S.I. Experiments were performed by T.S., Y.M., N.M. and J.T. T.S., J.T., P.N., N.I. and T.C.S. jointly analyzed and interpreted data.
Corresponding author
Ethics declarations
Competing interests
T.C.S., T.S., N.I. and J.T. hold rights under a United States patent entitled “Model mouse of Alzheimer's disease expressing FAD APP 716 and use thereof.” The patent number is 7,745,688. The authors, however, will provide academia with the mutant mice free of charge.
Integrated supplementary information
Supplementary Figure 1 Concept for generating new AD mouse models.
(a) Intrinsic problems of APP-Tg mice. The mice express non-physiologically high levels of APP. Because APP interacts with c-Jun N-terminal kinase interacting protein-1 (JIP-1), a mediator for kinesin-1, overexpressed APP may perturb axonal transport and generate artificial phenotype(s). APP fragments other than Aβ, i.e. sAPP, CTF-β and AICD, are also overproduced. Thus, it remains uncertain whether the various phenotypes observed in these mice are genuinely caused by Aβ deposition. See supplementary Table 1 for other problems. (b) Design of new mouse models. We humanized the mouse Aβ sequence and introduced Swedish and Beyreuther/Iberian mutations by knockin technology. We also generated mutant mice that in addition carried the Arctic mutation.
Supplementary Figure 2 Procedures to generate APPNL-F/NL-F mice.
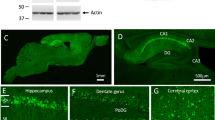
(a) APP wild-type allele, targeting vector and targeted allele. The mutations introduced to exons 16 and 17 are indicated by closed triangles. Restriction enzyme cleavage sites by ApaLI, NotI and XbaI are also shown. Neo denotes the neomycin-resistance gene cassette for positive selection surrounded by the lox/FRT sequence. SA and LA denote the short and long arms of the targeting vector, respectively. The annealing positions of the 5' and 3' probes used in the Southern blot analysis are located outside the targeted allele. (b) Mutations introduced to exons 16 and 17. Changes in the genomic sequence and in the amino acid sequence are depicted. Exon 16 contains the Swedish mutations and humanized sequence; exon 17 contains the Arctic (in case of APPNL-G-F/NL-G-F mice) and Beyreuther/Iberian mutations. Numbers represent amino acid positions in the Aβ sequence. (c) Germline transmission identified by Southern blot analysis. Genomic DNA extracted from an APPNL-F/NL-F mouse tail (clone #271) was digested by XbaI and ApaLI, and hybridized with the 5' and 3'probes shown in panel A. Southern blot analysis was repeated twice to select the positive clone for microinjection. (d) Genotyping of mice by PCR. The upper band is amplified from the wild-type allele, and the lower band from the mutant allele. (e) Northern blot analysis of APP mRNA. Total RNA extracted from brains was detected using a mouse APP-specific probe. β-actin was used as an internal control. We quantified the expression levels by densitometry and found no significant difference between the wild-type and mutant mice. Data represent mean ± s.e.m (n = 3). Each set of experiments was repeated at least three times to confirm the results. The full-length image are shown in Supplementary Figure 12.
Supplementary Figure 3 Western blot analyses and quantitative results of APP expression levels and neuroinflammation in APPNL-F mice.
(a) Western blot analyses of APP and APP-derived fragments in APPNL-F/NL-F mice. Antibodies to the N-terminus of APP (22C11), to the human sequence of Aβ (6E10), and to the C-terminus of APP were used. The full-length image are shown in Supplementary Figure 13. (b) Quantification of APP and its fragments in the brains of APPNL and APPNL-F KI mice. Intensities of immunoreactive bands on Western blots in a were quantified by densitometric analysis. We found no significant difference in the quantities of 22C11-reactive APP or AICD between the wild-type and the mutant mice (n = 6 of APPwt/wt, 6 of APPNL-F/wt, 6 of APPNL-F/NL-F, 4 of APPNL/wt and 4 of APPNL/NL, one-way ANOVA). Human Aβ sequence-containing (6E10-reactive) APP and CTF-β signals increased in a manner dependent on the Swedish mutation gene dose; CTF-α signals decreased. The levels of CTF-β and CTF-α in APPNL-F/NL-F mice were similar to those of APPNL/NL mice (n = 6 of APPwt/wt, 6 of APPNL-F/wt, 6 of APPNL-F/NL-F, 4 of APPNL/wt and 4 of APPNL/NL, one-way ANOVA with Sheffe's F test). Data represent mean ± s.e.m. (c) Cortical immunoreactivities of Iba1 and GFAP signals in 18-month-old APPwt/wt, APPNL/NL and APPNL-F/NL-F mice (Fig. 1d) were quantified. Data represent mean ± s.e.m. (n = 4 of APPwt/wt, 6 of APPNL-F/NL-F and 4 of APPNL/NL, one-way ANOVA with Sheffe's F test, **P < 0.01).
Supplementary Figure 4 Levels of APP, APP-derived fragments and Aβ in 2 month old APPNL, APPNL-F and APP23 mice.
(a) Western blot analyses of APP and APP-derived fragments in APPNL-F/NL-F and APP23 mice. See Fig. 1A legend for details. The full-length image are shown in Supplementary Figure 13b. (b–e) Steady-state levels of Aβ40 and Aβ42 and ratios of Aβ42/Aβ40 in the mouse brains. Panels B and C show the amounts of TS-soluble and GuHCl-extractable Aβ, respectively. Panels D and E show the ratio of Aβ42/Aβ40 in the TS and GuHCl fractions, respectively. Data represent mean ± s.e.m. (n = 5 of APPwt/wt, 4 of APPNL-F/wt, 5 of APPNL-F/NL-F, 4 of APPNL/wt and 4 of APPNL/NL and 3 of APP23, one-way ANOVA with Scheffe's F test, **P < 0.01).
Supplementary Figure 5 Initial deposition of Aβ in APPNL-F/NL-F mice.
(a) Immunohistchemical staining of Aβ plaques in APPNL-F/NL-F mice. Brain sections from 6-month-old APPNL-F/NL-F mice (n = 4) were immunostained using the anti-Aβ antibody, 6E10. The right panel shows an enlarged image of the area indicated by a square in the left panel. We detected approximately 1 to 2 plaques in an APPNL-F/NL-F cortical slice at this age. (b) N- and C-terminal structures of Aβ species in APPNL-F/NL-F brains. Brain sections from 6-month-old APPNL-F/NL-F mice (n = 4) were double stained using 4G8 antibody combined with antibodies to Aβ1–X, to Aβ3pE–X, to AβX–40 and to AβX–42. Scale bars represent 100 μm (a, left panel), 25 μm (a, right panel) and 50 μm (b), respectively.
Supplementary Figure 6 Aβ deposition in APP23 mice.
(a) Quantification of Aβ40 and Aβ42 in APP23 mouse brains. The amounts of GuHCl-extractable Aβ species at different ages are shown. (b) Aβ deposition in APP23 brain. Brain sections from 9-, 12- and 15-month-old APP23 mice were immunostained with the anti-Aβ antibody, 4G8. Immunoreactive Aβ deposition was detected from 12 months of age (n = 4/time point). Although the knockin mice start accumulating Aβ in cortex like in humans, APP23 mice simultaneously in cortex and hippocampus under the control of Thy-1 promoter. (c) N- and C-terminal structures of Aβ species in APP23 mouse brains. Serial sections from 18-month-old APP23 mouse brains (n = 4) were immunostained with antibodies to Aβ1–X, to Aβ3pE–X, to AβX–40 and to AβX–42. Scale bars represent 500 μm (b) and 100 μm (c).
Supplementary Figure 7 Aβ amyloidosis in heterozygous APPNL-F/wt mice.
Brain sections from 24- and 30-month-old mice were immunostained with anti-Aβ1–x antibody (n = 6). Aβ plaques were detected in the cortex of APPNL-F/wt mice over 24 months of age, whereas no plaques were detected in APPwt/wt and APPNL/NL mice (data not shown). Scale bars represent 200 μm.
Supplementary Figure 8 Aβ amyloidosis in sporadic AD brains.
(a) N- and C-terminal structures of Aβ species in sporadic AD brains. Serial cortical sections from sporadic AD patients (n = 4) were immunostained with the antibodies to Aβ1–X, to Aβ3pE–X, to AβX–40 and to AβX–42. (b) Neuroinflammation in sporadic AD brains. Inflammatory responses were detected by triple staining using FSB, anti-GFAP antibody and anti-Iba1 antibody as markers of cored Aβ plaques, astrocytosis and microgliosis, respectively. (c) Presynaptic alteration in sporadic AD. Brain sections were double-stained with 4G8 antibody and anti-synaptophysin antibody. (d) Postsynaptic alteration in sporadic AD. Brain sections were double-stained with 4G8 antibody and anti-PSD95 antibody. We observed synaptic disruptions at cored plaques but not at diffuse plaques. Scale bars represent 50 μm (a), 20 μm (b) and 10 μm (c, d), respectively.
Supplementary Figure 9 Phenotypes of calpastatin-deficient APPNL-F/NL-F mice.
(a) Effect of calpastatin deficiency on the survival of APPNL-F/NL-F, APPNL/NL and APP-Tg mice over 60 weeks of age. All the mouse lines were housed under identical conditions. (n = 22 of APPNL-F/NL-F, 24 of APPNL/NL, 25 of APPNL-F/NL-F x Cast–/– and 20 of APPNL/NL x Cast–/–) (b) Acceleration of Aβ deposition in APPNL-F/NL-F x Cast–/– brains. Brain sections from 15-month-old mice were immunostained using antibodies to different N-terminal structures of Aβ as indicated. Scale bars represent 500 μm. (c) Increased neuroinflammation in APPNL-F/NL-F x Cast–/– brains. Brain sections from 12-month-old mice were double-stained using anti-Iba1 and anti-GFAP antibodies. Cortical immunoreactivities were quantified as shown in the graph (n = 3, one-way ANOVA with Sheffe's F test, *P < 0.05 and **P < 0.01). Scale bars represent 100 μm. (d) Biochemical quantities of Aβ in APPNL-F/NL-F x Cast–/– brains. The levels of Aβ40 and Aβ42 in the TS and GuHCl fractions of cortex from 15-month-old mice were quantified by sandwich ELISA. Data represent mean ± s.e.m. (n = 5 of APPNL/NL, 5 of APPNL/NL x Cast–/–, 6 of APPNL-F/NL-F and 4 of APPNL-F/NL-F x Cast–/–, Student-t test, *P < 0.05 and **P < 0.01). (e) Memory impairment in APPNL-F/NL-F x Cast–/– mice. The Y-maze test was performed using 15-month-old mice. Data represent mean ± s.e.m. (n = 9 of APPNL/NL, 10 of APPNL/NL x Cast–/–, 9 of APPNL-F/NL-F and 11 of APPNL-F/NL-F x Cast–/–, one-way ANOVA with Sheffe's F test, *P < 0.05).
Supplementary Figure 10 Western blot analyses and quantitative results of APP expression levels and neuroinflammation in APPNL-G-F mice.
(a) Western blot analyses of APP and APP-derived fragments in APPNL-G-F/NL-G-F mice. See Figure 1A legend for details. The full-length image are shown in Supplementary Figure 13c. (b) Quantification of APP and its fragments in the brain of APPNL-G-F KI mice. Intensities of immunoreactive bands on Western blots shown in a were quantified by densitometric analysis. We found no significant difference in the quantities of 22C11-reactive APP or AICD between the wild-type and the mutant mice (n = 4, one-way ANOVA). Human Aβ sequence-containing (6E10-reactive) APP and CTF-β signals increased in a manner dependent on the Swedish mutation gene dose; CTF-α signals decreased. The levels of CTF-β and CTF-α in APPNL-G-F/NL-G-F mice were similar to those in APPNL-F/NL-F mice (n = 4, one-way ANOVA with Sheffe's F test). Consistent with the data presented in Supplementary Fig. 10d, the 6E10 immunoreactivity of APP in APPNL-G-F mice was smaller than that in APPNL-F mice (n = 4, one-way ANOVA with Sheffe's F test, **P < 0.01). Data represent mean ± s.e.m. (c) Cortical immunoreactivities of Iba1 and GFAP signals from 6-month-old APPwt/wt, APPNL-F/NL-F, APPNL-G-F/NL-G-F and 18-month-old APPNL-F/NL-F mice (Fig. 3d) were quantified. Data represent mean ± s.e.m. (n = 3 of APPwt/wt, 4 of APPNL-F/NL-F (6-month-old), 6 of APPNL-G-F/NL-G-F and 5 of APPNl-F/NL-F (18-month-old), one-way ANOVA with Sheffe's F test, *P < 0.05, **P < 0.01).
Supplementary Figure 11 Reactivity of antibodies to Arctic Aβ in APPNL-G-F/NL-G-F mice.
(a) Epitope map of anti-AP antibodies. (b) Quantification of Arctic AP spec1es usmg BNT77 as a capture antibody. BNT77 binds to the mid-portion of Aβ. A sandwich ELISA kit (Wako, Japan) was used. (c) Quantification of Arctic Aβ species using BAN50 as a capture antibody. BAN50 binds to the N-terminal region of Aβ. BNT77 and BAN50 captured Arctic Aβ more weakly than wild-type Aβ. (d) Immunohistochemistry using various anti-Aβ antibodies. Brain sections of 24-month-old APPNL-F/NL-F mice were immunostained using antibodies with different epitopes after antigen retrieval as indicated (upper panels); those of 9-month-old APPNL-G-F/NL-G-F mice were similarly immunostained (lower panels).
Supplementary Figure 12 Aβ deposition in heterozygous APPNL-G-F/wt mice.
(a) Quantification of Aβ40 and Aβ42 in heterozygous APPNL-G-F/wt mouse brains. Aβ40 and Aβ42 in the TS and GuHCl fractions of cortex from 2–12-month-old mice were quantified by ELISA using Arctic Aβ-based standard curves. BNT77 was used as a capture antibody. Data represent mean ± s.e.m. (n = 3, 4, 4, 4 and 4/indicated time point). (b) Aβ deposition in APPNL-G-F/wt brains. Brain sections from 2-, 4-, 6-, 9- and 12-month-old APPNL-G-F/wt mice were immunostained with anti-Aβx–42 antibody. Scale bars represent 500 μm. Plaque areas were quantified in a manner identical to that described in Figures 1 and 3. Data represent mean ± s.e.m. (n = 3, 4, 4, 4 and 4/indicated time point).
Supplementary Figure 14 The full-length images of Western blots.
(a) The full-length blot for Supplementary Figure 3a. (b) The full-length blot for Supplementary Figure 4a. (c) The full-length blot for Supplementary Figure 9a.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 and Supplementary Figures 1–14 (PDF 2596 kb)
Source data
Rights and permissions
About this article
Cite this article
Saito, T., Matsuba, Y., Mihira, N. et al. Single App knock-in mouse models of Alzheimer's disease. Nat Neurosci 17, 661–663 (2014). https://doi.org/10.1038/nn.3697
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3697
This article is cited by
-
Updates on mouse models of Alzheimer’s disease
Molecular Neurodegeneration (2024)
-
Dimethyl fumarate improves cognitive impairment and neuroinflammation in mice with Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
Identification of female-enriched and disease-associated microglia (FDAMic) contributes to sexual dimorphism in late-onset Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
The fluorescent ligand bTVBT2 reveals increased p-tau uptake by retinal microglia in Alzheimer’s disease patients and AppNL−F/NL−F mice
Alzheimer's Research & Therapy (2024)
-
Treatment with BRICHOS domain helps to clarify issues with Alzheimer mouse models
EMBO Molecular Medicine (2024)