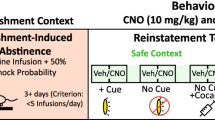
Abstract
The ventral pallidum is centrally positioned within mesocorticolimbic reward circuits, and its dense projection to the ventral tegmental area (VTA) regulates neuronal activity there. However, the ventral pallidum is a heterogeneous structure, and how this complexity affects its role within wider reward circuits is unclear. We found that projections to VTA from the rostral ventral pallidum (RVP), but not the caudal ventral pallidum (CVP), were robustly Fos activated during cue-induced reinstatement of cocaine seeking—a rat model of relapse in addiction. Moreover, designer receptor–mediated transient inactivation of RVP neurons, their terminals in VTA or functional connectivity between RVP and VTA dopamine neurons blocked the ability of drug-associated cues (but not a cocaine prime) to reinstate cocaine seeking. In contrast, CVP neuronal inhibition blocked cocaine-primed, but not cue-induced, reinstatement. This double dissociation in ventral pallidum subregional roles in drug seeking is likely to be important for understanding the mesocorticolimbic circuits underlying reward seeking and addiction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zahm, D.S. & Heimer, L. Two transpallidal pathways originating in the rat nucleus accumbens. J. Comp. Neurol. 302, 437–446 (1990).
Zahm, D.S., Williams, E. & Wohltmann, C. Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain. J. Comp. Neurol. 364, 340–362 (1996).
Kalivas, P.W., Churchill, L. & Klitenick, M.A. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience 57, 1047–1060 (1993).
Haber, S.N. & Nauta, W.J. Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience 9, 245–260 (1983).
Floresco, S.B., West, A.R., Ash, B., Moore, H. & Grace, A.A. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci. 6, 968–973 (2003).
Smith, K.S., Tindell, A.J., Aldridge, J.W. & Berridge, K.C. Ventral pallidum roles in reward and motivation. Behav. Brain Res. 196, 155–167 (2009).
Mogenson, G.J., Brudzynski, S.M., Wu, M., Yang, C.Y. & Yim, C.Y. From motivation to action: a review of dopaminergic regulation of limbic to nucleus accumbens to ventral pallidum to pedunculopontine nucleus circuitries involved in limbic-motor integration. in Limbic Motor Circuits in Neuropsychiatry (eds. Kalivas, P.W. & Barnes, C.D.) 193–236 (CRC Press, Boca Raton, Florida, 1993).
Churchill, L. & Kalivas, P.W. A topographically organized γ-aminobutyric acid projection from the ventral pallidum to the nucleus accumbens in the rat. J. Comp. Neurol. 345, 579–595 (1994).
Heimer, L., Zahm, D.S., Churchill, L., Kalivas, P.W. & Wohltmann, C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience 41, 89–125 (1991).
Kupchik, Y.M. & Kalivas, P.W. The rostral subcommissural ventral pallidum is a mix of ventral pallidal neurons and neurons from adjacent areas: an electrophysiological study. Brain Struct. Funct. 218, 1487–1500 (2013).
Root, D.H. et al. Differential roles of ventral pallidum subregions during cocaine self-administration behaviors. J. Comp. Neurol. 521, 558–588 (2013).
Yang, C.R. & Mogenson, G.J. Ventral pallidal neuronal responses to dopamine receptor stimulation in the nucleus accumbens. Brain Res. 489, 237–246 (1989).
McBride, W.J., Murphy, J.M. & Ikemoto, S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav. Brain Res. 101, 129–152 (1999).
Panagis, G., Miliaressis, E., Anagnostakis, Y. & Spyraki, C. Ventral pallidum self-stimulation: a moveable electrode mapping study. Behav. Brain Res. 68, 165–172 (1995).
Bengtson, C.P. & Osborne, P.B. Electrophysiological properties of cholinergic and noncholinergic neurons in the ventral pallidal region of the nucleus basalis in rat brain slices. J. Neurophysiol. 83, 2649–2660 (2000).
Zahm, D.S. & Heimer, L. Ventral striatopallidal parts of the basal ganglia in the rat: I. Neurochemical compartmentation as reflected by the distributions of neurotensin and substance P immunoreactivity. J. Comp. Neurol. 272, 516–535 (1988).
Johnson, P.I., Stellar, J.R. & Paul, A.D. Regional reward differences within the ventral pallidum are revealed by microinjections of a μ opiate receptor agonist. Neuropharmacology 32, 1305–1314 (1993).
Calder, A.J. et al. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur. J. Neurosci. 25, 3422–3428 (2007).
Smith, K.S. & Berridge, K.C. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J. Neurosci. 25, 8637–8649 (2005).
Grace, A.A., Floresco, S.B., Goto, Y. & Lodge, D.J. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 30, 220–227 (2007).
Tindell, A.J., Berridge, K.C. & Aldridge, J.W. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J. Neurosci. 24, 1058–1069 (2004).
Tachibana, Y. & Hikosaka, O. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron 76, 826–837 (2012).
Mahler, S.V. & Aston-Jones, G.S. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J. Neurosci. 32, 13309–13326 (2012).
Armbruster, B.N., Li, X., Pausch, M.H., Herlitze, S. & Roth, B.L. Evolving the lock to fit the key to create a family of G protein–coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 104, 5163–5168 (2007).
Ferguson, S.M. & Neumaier, J.F. Grateful DREADDs: engineered receptors reveal how neural circuits regulate behavior. Neuropsychopharmacology 37, 296–297 (2012).
Ungless, M.A. & Grace, A.A. Are you or aren't you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 35, 422–430 (2012).
Ikemoto, S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev. 56, 27–78 (2007).
Morales, M. & Pickel, V.M. Insights to drug addiction derived from ultrastructural views of the mesocorticolimbic system. Ann. NY Acad. Sci. 1248, 71–88 (2012).
Fields, H.L., Hjelmstad, G.O., Margolis, E.B. & Nicola, S.M. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu. Rev. Neurosci. 30, 289–316 (2007).
van Zessen, R., Phillips, J.L., Budygin, E.A. & Stuber, G.D. Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194 (2012).
Stamatakis, A.M. et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron 80, 1039–1053 (2013).
Hjelmstad, G.O., Xia, Y., Margolis, E.B. & Fields, H.L. Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons. J. Neurosci. 33, 6454–6459 (2013).
Witten, I.B. et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 72, 721–733 (2011).
Geisler, S., Derst, C., Veh, R.W. & Zahm, D.S. Glutamatergic afferents of the ventral tegmental area in the rat. J. Neurosci. 27, 5730–5743 (2007).
McFarland, K. & Kalivas, P.W. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 21, 8655–8663 (2001).
McFarland, K., Davidge, S.B., Lapish, C.C. & Kalivas, P.W. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci. 24, 1551–1560 (2004).
Robledo, P. & Koob, G.F. Two discrete nucleus accumbens projection areas differentially mediate cocaine self-administration in the rat. Behav. Brain Res. 55, 159–166 (1993).
Farrar, A.M. et al. Forebrain circuitry involved in effort-related choice: injections of the GABAA agonist muscimol into ventral pallidum alter response allocation in food-seeking behavior. Neuroscience 152, 321–330 (2008).
Colussi-Mas, J., Geisler, S., Zimmer, L., Zahm, D.S. & Berod, A. Activation of afferents to the ventral tegmental area in response to acute amphetamine: a double-labelling study. Eur. J. Neurosci. 26, 1011–1025 (2007).
Geisler, S. et al. Prominent activation of brainstem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacology 33, 2688–2700 (2008).
Childress, A.R. et al. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS ONE 3, e1506 (2008).
Zahm, D.S. Is the caudomedial shell of the nucleus accumbens part of the extended amygdala? A consideration of connections. Crit. Rev. Neurobiol. 12, 245–265 (1998).
Tripathi, A., Prensa, L. & Mengual, E. Axonal branching patterns of ventral pallidal neurons in the rat. Brain Struct. Funct. 218, 1133–1157 (2013).
Leung, B.K. & Balleine, B.W. The ventral striato-pallidal pathway mediates the effect of predictive learning on choice between goal-directed actions. J. Neurosci. 33, 13848–13860 (2013).
Robinson, M.J. & Berridge, K.C. Instant transformation of learned repulsion into motivational “wanting.”. Curr. Biol. 23, 282–289 (2013).
Lammel, S. et al. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57, 760–773 (2008).
Luo, A.H., Georges, F.E. & Aston-Jones, G.S. Novel neurons in ventral tegmental area fire selectively during the active phase of the diurnal cycle. Eur. J. Neurosci. 27, 408–422 (2008).
Robinson, T.E. & Berridge, K.C. Addiction. Annu. Rev. Psychol. 54, 25–53 (2003).
Beckley, J.T., Evins, C.E., Fedarovich, H., Gilstrap, M.J. & Woodward, J.J. Medial prefrontal cortex inversely regulates toluene-induced changes in markers of synaptic plasticity of mesolimbic dopamine neurons. J. Neurosci. 33, 804–813 (2013).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates (Academic Press/Elsevier, Amsterdam, Boston, 2006).
Acknowledgements
We thank P. Do, M.J. Gilstrap and E.C. Lin for assistance with behavioral testing and immunohistochemistry and B.L. Roth (Department of Pharmacology, University of North Carolina) for DREADD constructs and consultation on DREADDs. CNO was supplied by US National Institutes of Health (NIH) National Cancer Institute (NCI) under the auspices of NS064882-01 and by the National Institute of Mental Health (NIMH) Chemical Synthesis and Drug Supply Program. Research was supported by NIH grants F32 DA026692, K99 DA035251 (S.V.M.), F31 DA030891 (J.T.B.), R21 DA025837 (G.A.-J. and S.P.W.), R01 DA013951 (J.J.W.), R37 DA006214 and P50 DA015369 (G.A.-J.). This project was supported by the National Center for Research Resources and the Office of the Director of the National Institutes of Health through grant number C06 RR015455.
Author information
Authors and Affiliations
Contributions
S.V.M. attained funding, designed and conducted experiments, performed surgeries, analyzed data and wrote the manuscript. E.M.V. designed and conducted anesthetized electrophysiology experiments, analyzed these data and wrote the manuscript. J.T.B. designed and conducted slice electrophysiology experiments, analyzed these data and wrote the manuscript. C.R.K. conducted behavioral experiments and wrote the manuscript. E.M.M. performed surgeries for and conducted behavioral and immunohistochemical experiments. J.K. conducted immunohistochemical and confocal microscopy experiments. S.P.W. attained funding and contributed the Syn-GFP viral construct. K.D. contributed the TH∷Cre transgenic rat line. J.J.W. attained funding, designed and conducted slice electrophysiology experiments and wrote the manuscript. G.A-J. attained funding, designed experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Double dissociation for rostral vs. caudal VP in cued vs. primed reinstatement.
a) RVP, CVP, and surrounding structures are represented in the horizontal plane. Boxed area is enlarged in panels b&c. ac: anterior commissure; IPAC: interstitial nucleus of the posterior limb of the anterior commissure; Lat. Shell: lateral nucleus accumbens shell; LH: lateral hypothalamus; POA: preoptic hypothalamic area; SLEA: sublenticular extended amygdala. b) Top panel shows m±SEM active lever pressing during cue-induced reinstatement in animals with containment of hM4Di expression >95% within RVP or CVP, after vehicle or CNO (20 mg/kg, i.p.). *p=0.015. Bottom panel represents effects in individual animals of CNO microinjections into various parts of VP on cued reinstatement. Location of each oval represents site of virus injections in behaviorally tested animals (bilateral virus placement represented on a unilateral map; two ovals/animal). Size and shape of ovals represents the average zone of hM4Di expression around virus injection sites [m±SEM spread: dorso-ventral: m=1.18(0.02) mm; medio-lateral: m=0.72(0.02); rostro-caudal: m=0.95(0.02)]. Color of ovals represents change in cue-induced reinstatement after CNO injection (expressed as percentage of vehicle day lever pressing in the same animal). Blues indicate decreases from vehicle day due to hM4Di inhibition, yellows and reds represent increases in reinstatement responding over vehicle day. c) Using the same symbol logic as panel b, effects of CNO (20 mg/kg, i.p.) on cocaine-primed reinstatement are shown for RVP and CVP hM4Di animals. Bars=m±SEM. *p<0.05
Supplementary Figure 2 Cue-induced reinstatement elicits Fos in VTA-projecting neurons in RVP, not CVP.
a) Coronal 40 μm thick section from RVP (∼0.6 mm rostral of bregma) showing typical staining for Fos (blue-black nuclear stain) and CTb (brown somatic stain, indicating VTA-projecting neurons). Scale bar=1 mm. Expression typical of behaviorally tested CS+ group, n=8. b) Coronal 40 μm thick section from CVP (∼0.4 mm caudal of bregma) showing typical staining for Fos and CTb. Scale bar=1 mm. Expression typical of behaviorally tested CS+ group, n=8. c) Higher magnification view of Fos and CTb staining in RVP. Arrows indicate co-labeled cells. Scale bar=50 μm. d) Fos activation of VTA-projecting cells (%CTb cells that are Fos+; y-axis) in brain slices taken from various rostro-caudal levels of VP (mm rostral or caudal of bregma; x-axis). Symbols indicate the behavioral test prior to sacrifice (red diamonds: CS+ reinstatement, blue squares: control CS- cue, pink triangles: extinction test, black Xs: novel environment locomotor test). Note that the samples in which the greatest proportion of VTA-projecting neurons expressed Fos were in CS+ animals, and were located rostral of bregma (in RVP).
Supplementary Figure 3 Projection patterns of RVP and CVP to VTA.
a) RVP and CVP projections to rostral and caudal VTA are illustrated in the horizontal plane. b) Mean±SEM CTb+ cells/sample located in RVP (left) or CVP (right), projecting to either rostral or caudal VTA. CTb injections were localized to VTA either entirely rostral of the interpeduncular nucleus (blue bars), or entirely caudal of it (red bars). These animals also supplied data for a previous publication23, where additional details on CTb injection sites can be found. c) Mean±SEM total CTb+ cells/slice at various levels throughout the rostro-caudal axis of VP after CTb injection in VTA. Lines=m±SEM. d) Example of axonal labeling for the HA-tagged hM4Di receptor in midbrain after injection of Syn-hM4Di-HA-GFP into RVP. Strong axonal hM4Di expression (black) was seen in lateral VTA, and ventromedial SN (pars compacta). Scale bar=500 μm. Inset shows 60X magnification of axon terminals expressing HA (black) in the vicinity of VTA cells (red Nissl stained), scale bar=50 μm. The sample sizes of experimental groups with equivalent axonal DREADD expression in ventral midbrain are listed in Results. (e) Example of axonal labeling for the HA-tagged hM4Di receptor in midbrain after injection of Syn-hM4Di-HA-GFP into CVP. The strongest axonal hM4Di expression was seen in SN (especially pars reticulata), though fibers were also observed in VTA (inset). Scale bar=500 μm. Inset shows 60X magnification of axon terminals expressing HA (black) in the vicinity of VTA cells (red Nissl stained), scale bar=50 μm. The sample sizes of experimental groups with equivalent axonal DREADD expression in ventral midbrain are listed in Results.
Supplementary Figure 4 No changes in VTA dopamine neuron inputs caused by CNO in GFP control virus rats.
a) Syn-GFP control lentivirus used to express GFP under a neuronal-specific human synapsin promoter (Syn) in RVP. b) Representative traces from VTA dopamine neurons are shown in baseline conditions (left, black), and after CNO (right, red; scale=20 pA, 200 ms). c) CNO had no effect on mean sIPSC amplitude. d) CNO had no effect on mean sIPSC frequency. Lines=individual raw values.
Supplementary Figure 5 Midbrain CNO microinjection sites.
Midbrain cannulae placements for microinjections of CNO into VTA or SN in animals tested for reinstatement of cocaine seeking. Animals with placements within VTA borders are shown with filled black circles, animals with placements outside VTA (including in SN) are shown with unfilled (white) circles.
Supplementary Figure 6 Example hM4Di expression in VTA TH+ neurons and processes.
Confocal photomicrographs of VTA in TH::Cre rats after injection of DIO-Syn-hM4Di-mCherry. Equivalent expression seen in behavioral tested animals, n=9. Dopamine cells are labeled for TH (green, top left), and nearly all co-express the mCherry-tagged hM4Di receptor (red, bottom left). Yellow cells indicate cells and their processes co-expressing dopamine and DREADDs. Scale bar=50 μm.
Supplementary Figure 7 Disconnection of RVP from VTA or VTA Glutamate.
a) Animals received unilateral RVP Syn-hM4Di-HA-GFP, and a chronic cannula into contralateral VTA, and were tested for cue-induced reinstatement on separate days after i.p. 10 mg/kg CNO and intra-VTA vehicle (Veh-CNO; unilateral RVP inactivation control group), i.p. CNO plus unilateral intra-VTA cocktail of the AMPA/NMDA antagonists CNQX/AP5 (A/C-CNO: RVP/VTA glutamate disconnect group), or i.p. CNO plus unilateral intra-VTA cocktail of GABAA/B agonists baclofen and muscimol (B/M-CNO: RVP/VTA disconnect group). Some animals also received unilateral intra-VTA B/M + i.p. veh. (B/M-Veh: Unilateral VTA inactivation control group). b) Disconnection of RVP from VTA reduced reinstatement relative to unilateral RVP inhibition control, but neither unilateral VTA inactivation, nor disconnecting RVP from glutamate inputs did so. *p<0.05 Bars=m±SEM
Supplementary Figure 8 Inhibiting RVP inputs does not affect spontaneous excitatory inputs to VTA dopamine neurons.
a) Representative traces from VTA dopamine neurons are shown in baseline conditions (black), and b) after CNO (red; scale=20 pA, 200 ms). c) CNO had no effect on mean sIPSC amplitude. d) CNO had no effect on mean sIPSC frequency. Lines=individual raw values.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 7363 kb)
Rights and permissions
About this article
Cite this article
Mahler, S., Vazey, E., Beckley, J. et al. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci 17, 577–585 (2014). https://doi.org/10.1038/nn.3664
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3664
This article is cited by
-
Updating the striatal–pallidal wiring diagram
Nature Neuroscience (2024)
-
The secondary somatosensory cortex gates mechanical and heat sensitivity
Nature Communications (2024)
-
Dynamic Changes of the Infralimbic Cortex and Its Regulation of the Prelimbic Cortex in Rats with Chronic Inflammatory Pain
Neuroscience Bulletin (2024)
-
Astrocyte regulation of synaptic signaling in psychiatric disorders
Neuropsychopharmacology (2023)
-
A paraventricular thalamus to insular cortex glutamatergic projection gates “emotional” stress-induced binge eating in females
Neuropsychopharmacology (2023)