Abstract
Improved methods of noninvasively modulating human brain function are needed. Here we probed the influence of transcranial focused ultrasound (tFUS) targeted to the human primary somatosensory cortex (S1) on sensory-evoked brain activity and sensory discrimination abilities. The lateral and axial spatial resolution of the tFUS beam implemented were 4.9 mm and 18 mm, respectively. Electroencephalographic recordings showed that tFUS significantly attenuated the amplitudes of somatosensory evoked potentials elicited by median nerve stimulation. We also found that tFUS significantly modulated the spectral content of sensory-evoked brain oscillations. The changes produced by tFUS on sensory-evoked brain activity were abolished when the acoustic beam was focused 1 cm anterior or posterior to S1. Behavioral investigations showed that tFUS targeted to S1 enhanced performance on sensory discrimination tasks without affecting task attention or response bias. We conclude that tFUS can be used to focally modulate human cortical function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Thielscher, A., Opitz, A. & Windhoff, M. Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. Neuroimage 54, 234–243 (2011).
Opitz, A. et al. How the brain tissue shapes the electric field induced by transcranial magnetic stimulation. Neuroimage 58, 849–859 (2011).
Thielscher, A. & Kammer, T. Electric field properties of two commercial figure-8 coils in TMS: calculation of focality and efficiency. Clin. Neurophysiol. 115, 1697–1708 (2004).
Harvey, E.N. The effect of high frequency sound waves on heart muscle and other irritable tissues. Am. J. Physiol. 91, 284–290 (1929).
Mihran, R.T., Barnes, F.S. & Wachtel, H. Temporally-specific modification of myelinated axon excitability in vitro following a single ultrasound pulse. Ultrasound Med. Biol. 16, 297–309 (1990).
Tsui, P.H., Wang, S.H. & Huang, C.C. In vitro effects of ultrasound with different energies on the conduction properties of neural tissue. Ultrasonics 43, 560–565 (2005).
Tyler, W.J. et al. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE 3, e3511 (2008).
Bachtold, M.R., Rinaldi, P.C., Jones, J.P., Reines, F. & Price, L.R. Focused ultrasound modifications of neural circuit activity in a mammalian brain. Ultrasound Med. Biol. 24, 557–565 (1998).
Menz, M.D., Oralkan, O., Khuri-Yakub, P.T. & Baccus, S.A. Precise neural stimulation in the retina using focused ultrasound. J. Neurosci. 33, 4550–4560 (2013).
Tufail, Y. et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron 66, 681–694 (2010).
King, R.L., Brown, J.R., Newsome, W.T. & Pauly, K.B. Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med. Biol. 39, 312–331 (2013).
Shealy, C.N. & Henneman, E. Reversible effects of ultrasound on spinal reflexes. Arch. Neurol. 6, 374–386 (1962).
Manlapaz, J.S., Astroem, K.E., Ballantine, H.T. Jr. & Lele, P.P. Effects of ultrasonic radiation in experimental focal epilepsy in the cat. Exp. Neurol. 10, 345–356 (1964).
Min, B.K. et al. Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. BMC Neurosci. 12, 23 (2011).
Tufail, Y., Yoshihiro, A., Pati, S., Tauchmann, M.L. & Tyler, W.J. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat. Protoc. 6, 1453–1470 (2011).
Yoo, S.S. et al. Focused ultrasound modulates region-specific brain activity. Neuroimage 56, 1267–1275 (2011).
Fry, F.J., Ades, H.W. & Fry, W.J. Production of reversible changes in the central nervous system by ultrasound. Science 127, 83–84 (1958).
Deffieux, T. et al. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Curr. Biol. 23, 2430–2433 (2013).
Krasovitski, B., Frenkel, V., Shoham, S. & Kimmel, E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc. Natl. Acad. Sci. USA 108, 3258–3263 (2011).
Tyler, W.J. Noninvasive neuromodulation with ultrasound? A continuum mechanics hypothesis. Neuroscientist 17, 25–36 (2011).
White, P.J., Clement, G.T. & Hynynen, K. Local frequency dependence in transcranial ultrasound transmission. Phys. Med. Biol. 51, 2293–2305 (2006).
Hayner, M. & Hynynen, K. Numerical analysis of ultrasonic transmission and absorption of oblique plane waves through the human skull. J. Acoust. Soc. Am. 110, 3319–3330 (2001).
Allison, T. et al. Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short-latency activity. J. Neurophysiol. 62, 694–710 (1989).
Hari, R. & Forss, N. Magnetoencephalography in the study of human somatosensory cortical processing. Phil. Trans. R. Soc. Lond. B 354, 1145–1154 (1999).
Burton, H. & Sinclair, R.J. Attending to and remembering tactile stimuli: a review of brain imaging data and single-neuron responses. J. Clin. Neurophysiol. 17, 575–591 (2000).
Buzsáki, G. & Draguhn, A. Neuronal oscillations in cortical networks. Science 304, 1926–1929 (2004).
Makeig, S., Debener, S., Onton, J. & Delorme, A. Mining event-related brain dynamics. Trends Cogn. Sci. 8, 204–210 (2004).
Swets, J.A. Is there a sensory threshold? Science 134, 168–177 (1961).
Legon, W., Rowlands, A., Opitz, A., Sato, T.F. & Tyler, W.J. Pulsed ultrasound differentially stimulates somatosensory circuits in humans as indicated by EEG and FMRI. PLoS ONE 7, e51177 (2012).
Gavrilov, L.R., Tsirulnikov, E.M. & Davies, I.A. Application of focused ultrasound for the stimulation of neural structures. Ultrasound Med. Biol. 22, 179–192 (1996).
Bystritsky, A. et al. A review of low-intensity focused ultrasound pulsation. Brain Stimul. 4, 125–136 (2011).
Allison, T., Wood, C.C., McCarthy, G. & Spencer, D.D. Cortical somatosensory evoked potentials. II. Effects of excision of somatosensory or motor cortex in humans and monkeys. J. Neurophysiol. 66, 64–82 (1991).
Opitz, A. et al. Physiological observations validate finite element models for estimating subject-specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. Neuroimage 81, 253–264 (2013).
Hameroff, S. et al. Transcranial ultrasound (TUS) effects on mental states: a pilot study. Brain Stimul. 6, 409–415 (2013).
O'Reilly, M.A., Huang, Y. & Hynynen, K. The impact of standing wave effects on transcranial focused ultrasound disruption of the blood-brain barrier in a rat model. Phys. Med. Biol. 55, 5251–5267 (2010).
O'Brien, W.D. Jr. Ultrasound-biophysics mechanisms. Prog. Biophys. Mol. Biol. 93, 212–255 (2007).
Nyborg, W.L. Biological effects of ultrasound: development of safety guidelines. Part II: general review. Ultrasound Med. Biol. 27, 301–333 (2001).
Dalecki, D. Mechanical bioeffects of ultrasound. Annu. Rev. Biomed. Eng. 6, 229–248 (2004).
Leighton, T.G. What is ultrasound? Prog. Biophys. Mol. Biol. 93, 3–83 (2007).
Smith, P.L. & Ratcliff, R. Psychology and neurobiology of simple decisions. Trends Neurosci. 27, 161–168 (2004).
Romo, R. & Salinas, E. Flutter discrimination: neural codes, perception, memory and decision making. Nat. Rev. Neurosci. 4, 203–218 (2003).
Tyler, W.J. The mechanobiology of brain function. Nat. Rev. Neurosci. 13, 867–878 (2012).
Fukuda, M. et al. Short-latency median-nerve somatosensory-evoked potentials and induced gamma-oscillations in humans. Brain 131, 1793–1805 (2008).
Tallon-Baudry, C. & Bertrand, O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn. Sci. 3, 151–162 (1999).
Elias, W.J. et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 369, 640–648 (2013).
Windhoff, M., Opitz, A. & Thielscher, A. Electric field calculations in brain stimulation based on finite elements: an optimized processing pipeline for the generation and usage of accurate individual head models. Hum. Brain Mapp. 34, 923–935 (2013).
Goss, S.A., Johnston, R.L. & Dunn, F. Comprehensive compilation of empirical ultrasonic properties of mammalian tissues. J. Acoust. Soc. Am. 64, 423–457 (1978).
Legon, W., Rowlands, A., Opitz, A., Sato, T.F. & Tyler, W.J. Pulsed ultrasound differentially stimulates somatosensory circuits in humans as indicated by EEG and FMRI. PLoS ONE 7, e51177 (2012).
Tufail, Y., Yoshihiro, A., Pati, S., Tauchmann, M.L. & Tyler, W.J. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat. Protoc. 6, 1453–1470 (2011).
Delorme, A. & Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21 (2004).
Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190 (2007).
Makeig, S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr. Clin. Neurophysiol. 86, 283–293 (1993).
Macmillan, N.A. & Creelman, C.D. Detection Theory: A User's Guide 2nd edn. (Erlbaum, 2005).
Acknowledgements
Funding and equipment for this study was provided by a Technological Innovation Award from the McKnight Endowment for Neuroscience, Neurotrek, Inc. and the Virginia Tech Carilion Research Institute.
Author information
Authors and Affiliations
Contributions
W.L., T.F.S., A.O., J.M., A.B., A.W. and W.J.T. performed the experiments; W.L., T.F.S., A.O. and W.J.T. wrote the manuscript; W.L., T.F.S., A.O. and W.J.T. conducted the data analyses; W.J.T. supervised the project.
Corresponding author
Ethics declarations
Competing interests
W.J.T. is the cofounder of a medical device company. W.J.T., T.F.S. and A.O. are inventors on patent applications describing methods and devices for noninvasive brain stimulation.
Integrated supplementary information
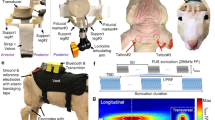
Supplementary Figure 1 Schematic outline of experimental approach.
a, The FUS transducer is shown placed at EEG electrode site CP3 on a realistic finite element model of a human head (left). A top-down schematic (right) illustrates transducer and electrode positioning at international 10-20 EEG electrode site sites C1, CP1, CP5 and P3. b, The timing strategy for delivering transcranial focused ultrasound (tFUS) or sham prior to, during, and following median nerve stimulation (MN stim) is illustrated. c, A schematic illustrates the pulsed ultrasound (US) waveform transmitted from the focused ultrasound transducer. The acoustic frequency of the waveform was 0.5 MHz and the pulse duration was 360 usec (black). The pulse repetition frequency (PRF; red) was 1 kHz for the stimulation duration of 500 msec.
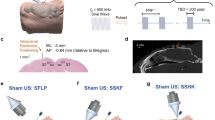
Supplementary Figure 2 Transcranial focused ultrasound does not affect task attention or response bias on somatosensory discrimination tasks.
a, Data acquired under sham (white) and tFUS (grey) treatments during two-point discrimination testing. The average percent correct for control catch trials (one pin) are illustrated as histograms (top). The criterion (c) data are illustrated as box plots (bottom) where the central line is the median and the edges of the box are the 25th and 75th percentiles with whiskers extending to the extreme data points. b, Data acquired under sham (white) and tFUS (grey) treatments during frequency discrimination testing. The average percent correct for control catch trials (no frequency difference between paired air puff stimuli) are illustrated as histograms (top). The criterion (c) data are illustrated as box plots (bottom) where the central line is the median and the edges of the box are the 25th and 75th percentiles with whiskers extending to the extreme data points.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 and Supplementary Tables 1–3 (PDF 591 kb)
Rights and permissions
About this article
Cite this article
Legon, W., Sato, T., Opitz, A. et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci 17, 322–329 (2014). https://doi.org/10.1038/nn.3620
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3620
This article is cited by
-
A review of functional neuromodulation in humans using low-intensity transcranial focused ultrasound
Biomedical Engineering Letters (2024)
-
Low-intensity transcranial ultrasound stimulation modulates neural activities in mice under propofol anaesthesia
BMC Neuroscience (2023)
-
Magnetothermal-based non-invasive focused magnetic stimulation for functional recovery in chronic stroke treatment
Scientific Reports (2023)
-
Reaching for the unreachable: low intensity focused ultrasound for non-invasive deep brain stimulation
Neuropsychopharmacology (2023)
-
Induction of a torpor-like hypothermic and hypometabolic state in rodents by ultrasound
Nature Metabolism (2023)