Abstract
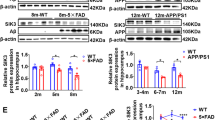
Expression of long-lasting synaptic plasticity and long-term memory requires protein synthesis, which can be repressed by phosphorylation of eukaryotic initiation factor 2 α-subunit (eIF2α). Elevated phosphorylation of eIF2α has been observed in the brains of Alzheimer's disease patients and Alzheimer's disease model mice. Therefore, we tested whether suppressing eIF2α kinases could alleviate synaptic plasticity and memory deficits in Alzheimer's disease model mice. Genetic deletion of eIF2α kinase PERK prevented enhanced phosphorylation of eIF2α and deficits in protein synthesis, synaptic plasticity and spatial memory in mice that express familial Alzheimer's disease–related mutations in APP and PSEN1. Similarly, deletion of another eIF2α kinase, GCN2, prevented impairments of synaptic plasticity and defects in spatial memory exhibited by the Alzheimer's disease model mice. Our findings implicate aberrant eIF2α phosphorylation as a previously unidentified molecular mechanism underlying Alzheimer's disease–related synaptic pathophysioloy and memory dysfunction and suggest that PERK and GCN2 are potential therapeutic targets for treatment of individuals with Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Holtzman, D.M., Morris, J.C. & Goate, A.M. Alzheimer's disease: the challenge of the second century. Sci. Transl. Med. 3, sr71 (2011).
Querfurth, H.W. & LaFerla, F.M. Alzheimer's disease. N. Engl. J. Med. 362, 329–344 (2010).
Selkoe, D.J. Resolving controversies on the path to Alzheimer's therapeutics. Nat. Med. 17, 1060–1065 (2011).
Haass, C. & Selkoe, D.J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 8, 101–112 (2007).
Costa-Mattioli, M., Sossin, W.S., Klann, E. & Sonenberg, N. Translational control of long-lasting synaptic plasticity and memory. Neuron 61, 10–26 (2009).
Klann, E. & Dever, T.E. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat. Rev. Neurosci. 5, 931–942 (2004).
Costa-Mattioli, M. et al. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature 436, 1166–1173 (2005).
Costa-Mattioli, M. et al. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 129, 195–206 (2007).
Jiang, Z. et al. eIF2alpha Phosphorylation-dependent translation in CA1 pyramidal cells impairs hippocampal memory consolidation without affecting general translation. J. Neurosci. 30, 2582–2594 (2010).
Klann, E., Antion, M.D., Banko, J.L. & Hou, L. Synaptic plasticity and translation initiation. Learn. Mem. 11, 365–372 (2004).
Wek, R.C., Jiang, H.-Y. & Anthony, T.G. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 34, 7–11 (2006).
Chang, R., Wong, A., Ng, H. & Hugon, J. Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer's disease. Neuroreport 13, 2429–2432 (2002).
Kim, S.M. et al. Activation of eukaryotic initiation factor-2 α-kinases in okadaic acid-treated neurons. Neuroscience 169, 1831–1839 (2010).
O'Connor, T. et al. Phosphorylation of the translation initiation factor eIF2α increases BACE1 levels and promotes amyloidogenesis. Neuron 60, 988–1009 (2008).
Jankowsky, J.L. et al. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol. Eng. 17, 157–165 (2001).
Zhang, P. et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell Biol. 22, 3864–3874 (2002).
Hoeffer, C.A. et al. Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron 60, 832–845 (2008).
Trinh, M.A. et al. Brain-specific disruption of the eIF2α kinase PERK decreases ATF4 expression and impairs behavioral flexibility. Cell Rep. 1, 676–688 (2012).
Schmidt, E.K., Clavarino, G., Ceppi, M. & Pierre, P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods 6, 275–277 (2009).
Cohen, E. et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell 139, 1157–1169 (2009).
Ma, T. et al. Amyloid β-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. J. Neurosci. 31, 5589–5595 (2011).
Palam, L.R., Baird, T.D. & Wek, R.C. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J. Biol. Chem. 286, 10939–10949 (2011).
Li, G., Scull, C., Ozcan, L. & Tabas, I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell Biol. 191, 1113–1125 (2010).
Wang, S. et al. Expression and functional profiling of neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme in prospectively studied elderly and Alzheimer's brain. J. Neurochem. 115, 47–57 (2010).
Sacktor, T.C. How does PKMζ maintain long-term memory? Nat. Rev. Neurosci. 12, 9–15 (2011).
Bramham, C.R. et al. The Arc of synaptic memory. Exp. Brain Res. 200, 125–140 (2010).
Lisman, J., Schulman, H. & Cline, H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 3, 175–190 (2002).
Leuba, G. et al. Differential changes in synaptic proteins in the Alzheimer frontal cortex with marked increase in PSD-95 postsynaptic protein. J. Alzheimers Dis. 15, 139–151 (2008).
Tsaytler, P. & Bertolotti, A. Exploiting the selectivity of protein phosphatase 1 for pharmacological intervention. FEBS J. 280, 766–770 (2013).
Cullinan, S.B. et al. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell Biol. 23, 7198–7209 (2003).
Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 (2012).
Zhang, P. et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell Biol. 22, 6681–6688 (2002).
Alberini, C. The role of protein synthesis during the labile phases of memory: revisiting the skepticism. Neurobiol. Learn. Mem. 89, 234–246 (2008).
Paschen, W., Proud, C.G. & Mies, G. Shut-down of translation, a global neuronal stress response: mechanisms and pathological relevance. Curr. Pharm. Des. 13, 1887–1902 (2007).
Wek, R.C. & Cavener, D.R. Translational control and the unfolded protein response. Antioxid. Redox Signal. 9, 2357–2371 (2007).
Lin, M.T. & Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 (2006).
Ma, T. & Klann, E. Amyloid β: linking synaptic plasticity failure to memory disruption in Alzheimer's disease. J. Neurochem. 120 (suppl. 1): 140–148 (2012).
Klann, E. & Richter, J.D. Translational control of synaptic plasticity and learning and memory. in Translational Control in Biology and Medicine (eds., Mathews, M.B., Sonenberg, N. & Hershey, J.W.B.) 485–506 (Cold Spring Harbor Laboratory Press, 2007).
Sidrauski, C. et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife 2, e00498 (2013).
Zhan, K., Narasimhan, J. & Wek, R.C. Differential activation of eIF2 kinases in response to cellular stresses in Schizosaccharomyces pombe. Genetics 168, 1867–1875 (2004).
Hamanaka, R.B., Bennett, B.S., Cullinan, S.B. & Diehl, J.A. PERK and GCN2 contribute to eIF2alpha phosphorylation and cell cycle arrest after activation of the unfolded protein response pathway. Mol. Biol. Cell 16, 5493–5501 (2005).
Jiang, H.-Y. et al. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol. Cell Biol. 24, 1365–1377 (2004).
Moreno, J.A. et al. Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature 485, 507–511 (2012).
Tsien, J.Z. et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell 87, 1317–1326 (1996).
Banko, J.L. et al. The translation repressor 4E–BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J. Neurosci. 25, 9581–9590 (2005).
Ma, T. et al. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer's disease. PLoS ONE 5, e12845 (2010).
Bonda, D.J. et al. Indoleamine 2,3-dioxygenase and 3-hydroxykynurenine modifications are found in the neuropathology of Alzheimer's disease. Redox Rep. 15, 161–168 (2010).
Barker, G.R.I. & Warburton, E.C. When is the hippocampus involved in recognition memory? J. Neurosci. 31, 10721–10731 (2011).
Acknowledgements
We thank the late Dr. Mark A. Smith (Case Western Reserve University) for providing postmortem Alzheimer's disease brain samples, R.C. Wek (Indiana University School of Medicine) for providing the ATF4 antibody, C.A. Hoeffer for help with the design of the mouse breeding, H. Bowling and M.V. Chao (New York University School of Medicine) for providing primary cultured neurons, E. Santini for advice on the statistical analyses for the mouse behavioral tests, Y. Chen for technical help, M. Dorsey for keeping colonies of transgenic mice, and all members of the Klann laboratory for comments on the manuscript. This work was supported by US National Institutes of Health grants NS034007 and NS047834, and Alzheimer's Association Investigator grant to E.K.
Author information
Authors and Affiliations
Contributions
T.M. did the majority of the experimental work and data analysis. M.A.T. performed western blots and immunohistochemistry on postmortem human tissue. A.J.W. did object location test scoring, performed western blots for the GCN2-deficient mice and genotyped mice. C.B. and E.G. performed western blots for the PKR mutant mice. P.P. provided puromycin antibody. P.P. and E.G. were involved in the experimental design. D.R.C. provided breeders of PERK conditional mutant and GCN2-deficient mice. E.K. directed and supervised the project. T.M. and E.K. designed the experiments and wrote the paper. All authors contributed to the analysis of data, discussion of the results and the final draft of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 1309 kb)
Rights and permissions
About this article
Cite this article
Ma, T., Trinh, M., Wexler, A. et al. Suppression of eIF2α kinases alleviates Alzheimer's disease–related plasticity and memory deficits. Nat Neurosci 16, 1299–1305 (2013). https://doi.org/10.1038/nn.3486
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3486
This article is cited by
-
Inhibition of PPP1R15A alleviates osteoporosis via suppressing RANKL-induced osteoclastogenesis
Acta Pharmacologica Sinica (2024)
-
Opposite modulation of functional recovery following contusive spinal cord injury in mice with oligodendrocyte-selective deletions of Atf4 and Chop/Ddit3
Scientific Reports (2023)
-
Synaptic proteasome is inhibited in Alzheimer’s disease models and associates with memory impairment in mice
Communications Biology (2023)
-
Effect of eIF2α in Neuronal Injury Induced by High Glucose and the Protective Mechanism of Resveratrol
Molecular Neurobiology (2023)
-
Downregulation of ATF-4 Attenuates the Endoplasmic Reticulum Stress–Mediated Neuroinflammation and Cognitive Impairment in Experimentally Induced Alzheimer’s Disease Model
Molecular Neurobiology (2023)