Abstract
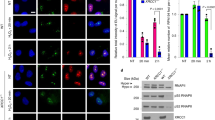
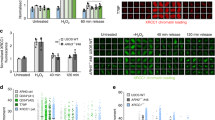
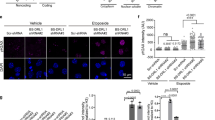
Defects in DNA repair have been linked to cognitive decline with age and neurodegenerative disease, yet the mechanisms that protect neurons from genotoxic stress remain largely obscure. We sought to characterize the roles of the NAD+-dependent deacetylase SIRT1 in the neuronal response to DNA double-strand breaks (DSBs). We found that SIRT1 was rapidly recruited to DSBs in postmitotic neurons, where it showed a synergistic relationship with ataxia telangiectasia mutated (ATM). SIRT1 recruitment to breaks was ATM dependent; however, SIRT1 also stimulated ATM autophosphorylation and activity and stabilized ATM at DSB sites. After DSB induction, SIRT1 also bound the neuroprotective class I histone deacetylase HDAC1. We found that SIRT1 deacetylated HDAC1 and stimulated its enzymatic activity, which was necessary for DSB repair through the nonhomologous end-joining pathway. HDAC1 mutations that mimic a constitutively acetylated state rendered neurons more susceptible to DNA damage, whereas pharmacological SIRT1 activators that promoted HDAC1 deacetylation also reduced DNA damage in two mouse models of neurodegeneration. We propose that SIRT1 is an apical transducer of the DSB response and that SIRT1 activation offers an important therapeutic avenue in neurodegeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
26 July 2013
In the version of this article initially published online, author Biafra Ahanonu's name was misspelled Ahononu. The error has been corrected for the print, PDF and HTML versions of this article.
References
Lu, T. et al. Gene regulation and DNA damage in the ageing human brain. Nature 429, 883–891 (2004).
Rass, U., Ahel, I. & West, S.C. Defective DNA repair and neurodegenerative disease. Cell 130, 991–1004 (2007).
Adamec, E., Vonsattel, J.P. & Nixon, R.A. DNA strand breaks in Alzheimer's disease. Brain Res. 849, 67–77 (1999).
Ferrante, R.J. et al. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J. Neurochem. 69, 2064–2074 (1997).
Nouspikel, T. & Hanawalt, P.C. When parsimony backfires: neglecting DNA repair may doom neurons in Alzheimer's disease. Bioessays 25, 168–173 (2003).
Gan, L. & Mucke, L. Paths of convergence: sirtuins in aging and neurodegeneration. Neuron 58, 10–14 (2008).
Kim, D. et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 26, 3169–3179 (2007).
Cruz, J.C., Tseng, H.C., Goldman, J.A., Shih, H. & Tsai, L.H. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron 40, 471–483 (2003).
Cruz, J.C. et al. p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid-β in vivo. J. Neurosci. 26, 10536–10541 (2006).
Kim, D. et al. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron 60, 803–817 (2008).
Singh, N.P., McCoy, M.T., Tice, R.R. & Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175, 184–191 (1988).
Seluanov, A., Mittelman, D., Pereira-Smith, O.M., Wilson, J.H. & Gorbunova, V. DNA end joining becomes less efficient and more error-prone during cellular senescence. Proc. Natl. Acad. Sci. USA 101, 7624–7629 (2004).
Berkovich, E., Monnat, R.J. Jr. & Kastan, M.B. Roles of ATM and NBS1 in chromatin structure modulation and DNA double-strand break repair. Nat. Cell Biol. 9, 683–690 (2007).
Berkovich, E., Monnat, R.J. Jr. & Kastan, M.B. Assessment of protein dynamics and DNA repair following generation of DNA double-strand breaks at defined genomic sites. Nat. Protoc. 3, 915–922 (2008).
Shiloh, Y. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3, 155–168 (2003).
Polo, S.E., Kaidi, A., Baskcomb, L., Galanty, Y. & Jackson, S.P. Regulation of DNA-damage responses and cell-cycle progression by the chromatin remodelling factor CHD4. EMBO J. 29, 3130–3139 (2010).
Qiu, Y. et al. HDAC1 acetylation is linked to progressive modulation of steroid receptor-induced gene transcription. Mol. Cell 22, 669–679 (2006).
Yang, T. et al. Acetylation of histone deacetylase 1 regulates NuRD corepressor complex activity. J. Biol. Chem. 287, 40279–40291 (2012).
Dai, H. et al. SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator. J. Biol. Chem. 285, 32695–32703 (2010).
Luo, Y. et al. Trans-regulation of histone deacetylase activities through acetylation. J. Biol. Chem. 284, 34901–34910 (2009).
Miller, K.M. et al. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat. Struct. Mol. Biol. 17, 1144–1151 (2010).
Yoshiyama, Y. et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007).
Lee, J.H. & Paull, T.T. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene 26, 7741–7748 (2007).
Yuan, Z., Zhang, X., Sengupta, N., Lane, W.S. & Seto, E. SIRT1 regulates the function of the Nijmegen breakage syndrome protein. Mol. Cell 27, 149–162 (2007).
Sun, Y., Jiang, X., Chen, S., Fernandes, N. & Price, B.D. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc. Natl. Acad. Sci. USA 102, 13182–13187 (2005).
Ball, A.R. Jr. & Yokomori, K. Damage site chromatin: open or closed? Curr. Opin. Cell Biol. 23, 277–283 (2011).
Yuan, Z.M. et al. Role for p300 in stabilization of p53 in the response to DNA damage. J. Biol. Chem. 274, 1883–1886 (1999).
Ikura, T. et al. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell 102, 463–473 (2000).
Sykes, S.M. et al. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol. Cell 24, 841–851 (2006).
Bouras, T. et al. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J. Biol. Chem. 280, 10264–10276 (2005).
Peng, L. et al. SIRT1 negatively regulates the activities, functions, and protein levels of hMOF and TIP60. Mol. Cell. Biol. 32, 2823–2836 (2012).
Yankner, B.A., Lu, T. & Loerch, P. The aging brain. Annu. Rev. Pathol. 3, 41–66 (2008).
Gao, J. et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466, 1105–1109 (2010).
Oberdoerffer, P. et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 135, 907–918 (2008).
Montgomery, R.L. et al. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 21, 1790–1802 (2007).
Cheng, H.L. et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. USA 100, 10794–10799 (2003).
Yang, X.J. & Seto, E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat. Rev. Mol. Cell Biol. 9, 206–218 (2008).
Takamiya, K., Mao, L., Huganir, R.L. & Linden, D.J. The glutamate receptor–interacting protein family of GluR2-binding proteins is required for long-term synaptic depression expression in cerebellar Purkinje cells. J. Neurosci. 28, 5752–5755 (2008).
Davis, J.B. & Maher, P. Protein kinase C activation inhibits glutamate-induced cytotoxicity in a neuronal cell line. Brain Res. 652, 169–173 (1994).
Vonesch, C. & Unser, M. A fast thresholded landweber algorithm for wavelet-regularized multidimensional deconvolution. IEEE Trans. Image Process. 17, 539–549 (2008).
Carpenter, A.E. et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 (2006).
Dundr, M. & Misteli, T. Measuring dynamics of nuclear proteins by photobleaching. Curr. Protoc. Cell Biol. Chapter 13, Unit 13.5 (2003).
Snapp, E.L., Altan, N. & Lippincott-Schwartz, J. Measuring protein mobility by photobleaching GFP chimeras in living cells. Curr. Protoc. Cell Biol. Chapter 21, Unit 21.1 (2003).
Roy, A., Kucukural, A. & Zhang, Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 (2010).
Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9, 40 (2008).
Finnin, M.S. et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 401, 188–193 (1999).
Kruhlak, M.J., Celeste, A. & Nussenzweig, A. Monitoring DNA breaks in optically highlighted chromatin in living cells by laser scanning confocal microscopy. Methods Mol. Biol. 523, 125–140 (2009).
Nowak, D.E., Tian, B. & Brasier, A.R. Two-step cross-linking method for identification of NF-κB gene network by chromatin immunoprecipitation. Biotechniques 39, 715–725 (2005).
Zeng, P.Y., Vakoc, C.R., Chen, Z.C., Blobel, G.A. & Berger, S.L. In vivo dual cross-linking for identification of indirect DNA-associated proteins by chromatin immunoprecipitation. Biotechniques 41, 694, 696, 698 (2006).
Haumaitre, C., Lenoir, O. & Scharfmann, R. Histone deacetylase inhibitors modify pancreatic cell fate determination and amplify endocrine progenitors. Mol. Cell Bio. 28, 6373–6383 (2008).
Zhao, Y., Zhang, W. & White, M.A. Capillary high-performance liquid chromatography/mass spectrometric analysis of proteins from affinity-purified plasma membrane. Anal. Chem. 75, 3751–3757 (2003).
Acknowledgements
We thank F.W. Alt (Harvard Medical School) and E.N. Olson (University of Texas Southwestern Medical Center) for providing the Sirt1loxP/loxP and Hdac1loxP/loxP mice, respectively; R. Huganir (Johns Hopkins University) for providing the lentiviral Cre constructs; V. Suri, J. Ellis and G. Vlasuk (Sirtris) for providing compound #10 and, together with J. Gräff, for critical comments on the manuscript; M. Kastan (St. Jude's Medical Research Hospital) for gifting the I-PpoI-ER construct; and V. Gorbunova (University of Rochester) for providing the NHEJ constructs. This work was supported by funding from US National Institutes of Health (NIH) PO1 grant AG27916, the Howard Hughes Medical Institute, the Neurodegeneration Consortium and the Glenn award for research in biological mechanisms of aging to L.-H.T., NIH grant R01 HL095674 to Y.Q. and NIH grant U54 RR020389 to Y.Z. M.M.D. was supported by NIH training grants T32 GM007484 and T32 MH081728.
Author information
Authors and Affiliations
Contributions
This study was designed by M.M.D., R.M. and L.-H.T. and was directed and coordinated by L.-H.T. M.M.D. and R.M. planned and performed most of the experiments. L.P. maintained the Sirt1loxP/loxP and Hdac1loxP/loxP mice and, together with B.A., helped with the microirradiation experiments. Y.C. and Y.Z. conducted the mass spectrometry analysis of HDAC1 acetylation. D.K. and J.G. performed some preliminary experiments with CK-p25 mice, and J.G. performed the compound #10 treatment and subsequent analysis of CK-p25 mice. P.-C.P. contributed to statistical analysis and quantification of several experiments, and Y.Q. developed and provided the antibody to acetylated HDAC1 Lys432. R.M., M.M.D. and L.-H.T. wrote the manuscript with critical input from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures and Text
Supplementary Figures 1–5 (PDF 9691 kb)
Rights and permissions
About this article
Cite this article
Dobbin, M., Madabhushi, R., Pan, L. et al. SIRT1 collaborates with ATM and HDAC1 to maintain genomic stability in neurons. Nat Neurosci 16, 1008–1015 (2013). https://doi.org/10.1038/nn.3460
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3460
This article is cited by
-
CYLD induces high oxidative stress and DNA damage through class I HDACs to promote radiosensitivity in nasopharyngeal carcinoma
Cell Death & Disease (2024)
-
Alzheimer’s Disease-Related Epigenetic Changes: Novel Therapeutic Targets
Molecular Neurobiology (2024)
-
C9orf72 functions in the nucleus to regulate DNA damage repair
Cell Death & Differentiation (2023)
-
Human ALS/FTD brain organoid slice cultures display distinct early astrocyte and targetable neuronal pathology
Nature Neuroscience (2021)
-
SIRT1 coordinates with the CRL4B complex to regulate pancreatic cancer stem cells to promote tumorigenesis
Cell Death & Differentiation (2021)