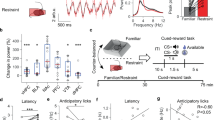
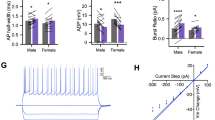
Abstract
Stressors affect dopamine-dependent behaviors such as motivation, although the underlying neurobiological mechanism is not well defined. We report that corticotropin-releasing factor (CRF) acts in the ventral tegmental area (VTA) to reduce the motivation to work for food rewards. CRF in the VTA regulates dopamine output in a stimulus- and pathway-specific manner, offering a mechanism by which acute stress selectively regulates information transmission via the VTA to reprioritize motivated behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sinha, R. Psychopharmacology (Berl.) 158, 343–359 (2001).
Meyer, S.E., Chrousos, G.P. & Gold, P.W. Dev. Psychopathol. 13, 565–580 (2001).
Salamone, J.D., Correa, M., Farrar, A.M., Nunes, E.J. & Pardo, M. Front. Behav. Neurosci. 3, doi:10.3389/neuro.08.013 (2009).
Roitman, M.F., Stuber, G.D., Phillips, P.E., Wightman, R.M. & Carelli, R.M. J. Neurosci. 24, 1265–1271 (2004).
Phillips, P.E., Stuber, G.D., Heien, M.L., Wightman, R.M. & Carelli, R.M. Nature 422, 614–618 (2003).
Inglis, F.M. & Moghaddam, B. J. Neurochem. 72, 1088–1094 (1999).
Tidey, J.W. & Miczek, K.A. Brain Res. 721, 140–149 (1996).
Wang, B. et al. J. Neurosci. 25, 5389–5396 (2005).
Beckstead, M.J. et al. Neuropsychopharmacology 34, 1926–1935 (2009).
Ungless, M.A. et al. Neuron 39, 401–407 (2003).
Wanat, M.J., Hopf, F.W., Stuber, G.D., Phillips, P.E. & Bonci, A. J. Physiol. (Lond.) 586, 2157–2170 (2008).
Wanat, M.J., Kuhnen, C.M. & Phillips, P.E. J. Neurosci. 30, 12020–12027 (2010).
Georges, F. & Aston-Jones, G. J. Neurosci. 22, 5173–5187 (2002).
Scarnati, E., Campana, E. & Pacitti, C. Brain Res. 304, 351–361 (1984).
Zweifel, L.S. et al. Proc. Natl. Acad. Sci. USA 106, 7281–7288 (2009).
Zacharko, R.M. & Anisman, H. Neurosci. Biobehav. Rev. 15, 391–405 (1991).
Shafiei, N., Gray, M., Viau, V. & Floresco, S.B. Neuropsychopharmacology 37, 2194–2209 (2012).
Cabib, S. & Puglisi-Allegra, S. Psychopharmacology (Berl.) 128, 331–342 (1996).
Lemos, J.C. et al. Nature 490, 402–406 (2012).
Blacktop, J.M. et al. J. Neurosci. 31, 11396–11403 (2011).
Kalivas, P.W., Duffy, P. & Latimer, L.G. J. Pharmacol. Exp. Ther. 242, 757–763 (1987).
Clark, J.J. et al. Nat. Methods 7, 126–129 (2010).
Heien, M.L. et al. Proc. Natl. Acad. Sci. USA 102, 10023–10028 (2005).
Acknowledgements
We would like to thank S. Ng-Evans for invaluable technical support and C. Akers for technical assistance. We thank N. Hollon, J. Clark and S. Sandberg for scientific discussion. This work was funded by the US National Institutes of Health (R01-MH079292, P.E.M.P.; R01-DA016782, A.B. and P.E.M.P.; T32-AA009455 and F32-DA026273, M.J.W.) and NARSAD (P.E.M.P.).
Author information
Authors and Affiliations
Contributions
M.J.W., A.B. and P.E.M.P. designed the experiments. M.J.W. collected and analyzed the data. M.J.W. and P.E.M.P. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9, Supplementary Table 1 (PDF 12066 kb)
Rights and permissions
About this article
Cite this article
Wanat, M., Bonci, A. & Phillips, P. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat Neurosci 16, 383–385 (2013). https://doi.org/10.1038/nn.3335
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3335
This article is cited by
-
Chemogenetic activation of corticotropin-releasing factor-expressing neurons in the anterior bed nucleus of the stria terminalis reduces effortful motivation behaviors
Neuropsychopharmacology (2024)
-
Chronically dysregulated corticosterone impairs dopaminergic transmission in the dorsomedial striatum by sex-divergent mechanisms
Neuropsychopharmacology (2023)
-
CRHCeA→VTA inputs inhibit the positive ensembles to induce negative effect of opiate withdrawal
Molecular Psychiatry (2021)
-
Ventral tegmental area GABA neurons mediate stress-induced blunted reward-seeking in mice
Nature Communications (2021)
-
Daidzein modulates cocaine-reinforcing effects and cue-induced cocaine reinstatement in CD-1 male mice
Psychopharmacology (2021)