Abstract
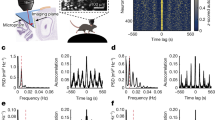
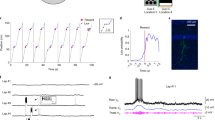
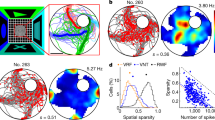
Neurons in the medial entorhinal cortex exhibit a grid-like spatial pattern of spike rates that has been proposed to represent a neural code for path integration. To understand how grid cell firing arises from the combination of intrinsic conductances and synaptic input in medial entorhinal stellate cells, we performed patch-clamp recordings in mice navigating in a virtual-reality environment. We found that the membrane potential signature of stellate cells during firing field crossings consisted of a slow depolarization driving spike output. This was best predicted by network models in which neurons receive sustained depolarizing synaptic input during a field crossing, such as continuous attractor network models of grid cell firing. Another key feature of the data, phase precession of intracellular theta oscillations and spiking with respect to extracellular theta oscillations, was best captured by an oscillatory interference model. Thus, these findings provide crucial new information for a quantitative understanding of the cellular basis of spatial navigation in the entorhinal cortex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hafting, T., Fyhn, M., Molden, S., Moser, M.B. & Moser, E.I. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806 (2005).
Sargolini, F. et al. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312, 758–762 (2006).
Fyhn, M., Hafting, T., Witter, M.P., Moser, E.I. & Moser, M.B. Grid cells in mice. Hippocampus 18, 1230–1238 (2008).
Hafting, T., Fyhn, M., Bonnevie, T., Moser, M.B. & Moser, E.I. Hippocampus-independent phase precession in entorhinal grid cells. Nature 453, 1248–1252 (2008).
Reifenstein, E.T., Kempter, R., Schreiber, S., Stemmler, M.B. & Herz, A.V. Grid cells in rat entorhinal cortex encode physical space with independent firing fields and phase precession at the single-trial level. Proc. Natl. Acad. Sci. USA 109, 6301–6306 (2012).
Fuhs, M.C. & Touretzky, D.S. A spin glass model of path integration in rat medial entorhinal cortex. J. Neurosci. 26, 4266–4276 (2006).
McNaughton, B.L., Battaglia, F.P., Jensen, O., Moser, E.I. & Moser, M.B. Path integration and the neural basis of the 'cognitive map'. Nat. Rev. Neurosci. 7, 663–678 (2006).
Burgess, N. Grid cells and theta as oscillatory interference: theory and predictions. Hippocampus 18, 1157–1174 (2008).
Burgess, N., Barry, C. & O'Keefe, J. An oscillatory interference model of grid cell firing. Hippocampus 17, 801–812 (2007).
Hasselmo, M.E., Giocomo, L.M. & Zilli, E.A. Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons. Hippocampus 17, 1252–1271 (2007).
Navratilova, Z., Giocomo, L.M., Fellous, J.M., Hasselmo, M.E. & McNaughton, B.L. Phase precession and variable spatial scaling in a periodic attractor map model of medial entorhinal grid cells with realistic after-spike dynamics. Hippocampus 22, 772–789 (2012).
Burak, Y. & Fiete, I.R. Accurate path integration in continuous attractor network models of grid cells. PLoS Comput. Biol. 5, e1000291 (2009).
Hölscher, C., Schnee, A., Dahmen, H., Setia, L. & Mallot, H.A. Rats are able to navigate in virtual environments. J. Exp. Biol. 208, 561–569 (2005).
Harvey, C.D., Collman, F., Dombeck, D.A. & Tank, D.W. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946 (2009).
Brun, V.H. et al. Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex. Hippocampus 18, 1200–1212 (2008).
Alonso, A. & Klink, R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J. Neurophysiol. 70, 128–143 (1993).
Garden, D.L., Dodson, P.D., O'Donnell, C., White, M.D. & Nolan, M.F. Tuning of synaptic integration in the medial entorhinal cortex to the organization of grid cell firing fields. Neuron 60, 875–889 (2008).
Giocomo, L.M., Zilli, E.A., Fransén, E. & Hasselmo, M.E. Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing. Science 315, 1719–1722 (2007).
Fernandez, F.R. & White, J.A. Artificial synaptic conductances reduce subthreshold oscillations and periodic firing in stellate cells of the entorhinal cortex. J. Neurosci. 28, 3790–3803 (2008).
Nolan, M.F., Dudman, J.T., Dodson, P.D. & Santoro, B. HCN1 channels control resting and active integrative properties of stellate cells from layer II of the entorhinal cortex. J. Neurosci. 27, 12440–12451 (2007).
Quilichini, P., Sirota, A. & Buzsáki, G. Intrinsic circuit organization and theta-gamma oscillation dynamics in the entorhinal cortex of the rat. J. Neurosci. 30, 11128–11142 (2010).
Welday, A.C., Shlifer, I.G., Bloom, M.L., Zhang, K. & Blair, H.T. Cosine directional tuning of theta cell burst frequencies: evidence for spatial coding by oscillatory interference. J. Neurosci. 31, 16157–16176 (2011).
Zilli, E.A. & Hasselmo, M.E. Coupled noisy spiking neurons as velocity-controlled oscillators in a model of grid cell spatial firing. J. Neurosci. 30, 13850–13860 (2010).
Pastoll, H., Solanka, L., van Rossum, M.C. & Nolan, M.F. Feedback inhibition enables theta-nested gamma oscillations and grid firing fields. Neuron 77, 141–154 (2013).
Couey, J.J. et al. Recurrent inhibitory circuitry as a mechanism for grid formation. Nat. Neurosci. 10.1038/nn.3310 (2013).
Mehta, M.R., Lee, A.K. & Wilson, M.A. Role of experience and oscillations in transforming a rate code into a temporal code. Nature 417, 741–746 (2002).
Burgess, N. & O'Keefe, J. Models of place and grid cell firing and theta rhythmicity. Curr. Opin. Neurobiol. 21, 734–744 (2011).
Koenig, J., Linder, A.N., Leutgeb, J.K. & Leutgeb, S. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science 332, 592–595 (2011).
Brandon, M.P. et al. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science 332, 595–599 (2011).
Haas, J.S. & White, J.A. Frequency selectivity of layer II stellate cells in the medial entorhinal cortex. J. Neurophysiol. 88, 2422–2429 (2002).
Kropff, E. & Treves, A. The emergence of grid cells: intelligent design or just adaptation? Hippocampus 18, 1256–1269 (2008).
Zilli, E.A. Models of grid cell spatial firing published 2005–2011. Front. Neural Circuits 6, 16 (2012).
O'Keefe, J. & Recce, M.L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330 (1993).
Hasselmo, M.E. & Brandon, M.P. A model combining oscillations and attractor dynamics for generation of grid cell firing. Front. Neural Circuits 6, 30 (2012).
Yartsev, M.M., Witter, M.P. & Ulanovsky, N. Grid cells without theta oscillations in the entorhinal cortex of bats. Nature 479, 103–107 (2011).
Dodson, P.D., Pastoll, H. & Nolan, M.F. Dorsal-ventral organization of theta-like activity intrinsic to entorhinal stellate neurons is mediated by differences in stochastic current fluctuations. J. Physiol. (Lond.) 589, 2993–3008 (2011).
Bourke, P. Spherical mirror: a new approach to hemispherical dome projection. Planetarian 34, 6–9 (2005).
Wagor, E., Mangini, N.J. & Pearlman, A.L. Retinotopic organization of striate and extrastriate visual cortex in the mouse. J. Comp. Neurol. 193, 187–202 (1980).
Margrie, T.W., Brecht, M. & Sakmann, B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflügers Arch. 444, 491–498 (2002).
Klink, R. & Alonso, A. Morphological characteristics of layer II projection neurons in the rat medial entorhinal cortex. Hippocampus 7, 571–583 (1997).
Kempter, R., Leibold, C., Buzsáki, G., Diba, K. & Schmidt, R. Quantifying circular-linear associations: hippocampal phase precession. J. Neurosci. Methods 207, 113–124 (2012).
Carnevale, N.T. & Hines, M.L. The NEURON Book (Cambridge University Press, 2006).
Fransén, E., Alonso, A.A., Dickson, C.T., Magistretti, J. & Hasselmo, M.E. Ionic mechanisms in the generation of subthreshold oscillations and action potential clustering in entorhinal layer II stellate neurons. Hippocampus 14, 368–384 (2004).
Katz, Y. et al. Synapse distribution suggests a two-stage model of dendritic integration in CA1 pyramidal neurons. Neuron 63, 171–177 (2009).
Acknowledgements
We are grateful to N. Burgess, B. Clark, P. Dayan, K. Harris, P. Latham, J. O'Keefe, A. Packer, A. Roth, S. Turaga and C. Wilms for helpful discussions and for comments on the manuscript, and to A. Naeem for assistance with histology. This work was supported by grants from the Wellcome Trust, European Research Council and Gatsby Charitable Foundation, and by a fellowship to C. S.-H. from the Alexander von Humboldt Foundation.
Author information
Authors and Affiliations
Contributions
C.S.-H. and M.H. designed the study, interpreted the results and wrote the paper. C.S.-H. performed the experiments, analysis and modeling.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Table 1 (PDF 13164 kb)
Supplementary Video 1
Video of mouse navigation in virtual reality (MP4 5403 kb)
Rights and permissions
About this article
Cite this article
Schmidt-Hieber, C., Häusser, M. Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nat Neurosci 16, 325–331 (2013). https://doi.org/10.1038/nn.3340
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3340
This article is cited by
-
Ketamine evoked disruption of entorhinal and hippocampal spatial maps
Nature Communications (2023)
-
Phase information is conserved in sparse, synchronous population-rate-codes via phase-to-rate recoding
Nature Communications (2023)
-
A synaptic signal for novelty processing in the hippocampus
Nature Communications (2022)
-
Presynaptic endoplasmic reticulum regulates short-term plasticity in hippocampal synapses
Communications Biology (2021)
-
Perineuronal nets stabilize the grid cell network
Nature Communications (2021)