Abstract
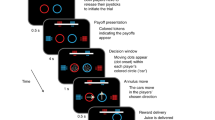
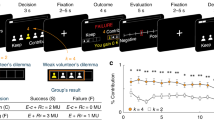
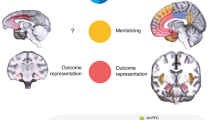
Social decisions are crucial for the success of individuals and the groups that they comprise. Group members respond vicariously to benefits obtained by others, and impairments in this capacity contribute to neuropsychiatric disorders such as autism and sociopathy. We examined the manner in which neurons in three frontal cortical areas encoded the outcomes of social decisions as monkeys performed a reward-allocation task. Neurons in the orbitofrontal cortex (OFC) predominantly encoded rewards that were delivered to oneself. Neurons in the anterior cingulate gyrus (ACCg) encoded reward allocations to the other monkey, to oneself or to both. Neurons in the anterior cingulate sulcus (ACCs) signaled reward allocations to the other monkey or to no one. In this network of received (OFC) and foregone (ACCs) reward signaling, ACCg emerged as an important nexus for the computation of shared experience and social reward. Individual and species-specific variations in social decision-making might result from the relative activation and influence of these areas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Fehr, E. & Fischbacher, U. The nature of human altruism. Nature 425, 785–791 (2003).
Gallese, V., Keysers, C. & Rizzolatti, G. A unifying view of the basis of social cognition. Trends Cogn. Sci. 8, 396–403 (2004).
Berger, S.M. Conditioning through vicarious instigation. Psychol. Rev. 69, 450–466 (1962).
Rilling, J. et al. A neural basis for social cooperation. Neuron 35, 395–405 (2002).
de Quervain, D.J. et al. The neural basis of altruistic punishment. Science 305, 1254–1258 (2004).
Baron-Cohen, S., Leslie, A.M. & Frith, U. Does the autistic child have a “theory of mind”? Cognition 21, 37–46 (1985).
Adolphs, R. Cognitive neuroscience of human social behavior. Nat. Rev. Neurosci. 4, 165–178 (2003).
Behrens, T.E., Hunt, L.T. & Rushworth, M.F. The computation of social behavior. Science 324, 1160–1164 (2009).
Rudebeck, P.H., Buckley, M.J., Walton, M.E. & Rushworth, M.F. A role for the macaque anterior cingulate gyrus in social valuation. Science 313, 1310–1312 (2006).
Kennerley, S.W., Walton, M.E., Behrens, T.E., Buckley, M.J. & Rushworth, M.F. Optimal decision making and the anterior cingulate cortex. Nat. Neurosci. 9, 940–947 (2006).
Hayden, B.Y., Pearson, J.M. & Platt, M.L. Fictive reward signals in the anterior cingulate cortex. Science 324, 948–950 (2009).
Tremblay, L. & Schultz, W. Relative reward preference in primate orbitofrontal cortex. Nature 398, 704–708 (1999).
Padoa-Schioppa, C. & Assad, J.A. Neurons in the orbitofrontal cortex encode economic value. Nature 441, 223–226 (2006).
Schoenbaum, G., Roesch, M.R., Stalnaker, T.A. & Takahashi, Y.K. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat. Rev. Neurosci. 10, 885–892 (2009).
Kennerley, S.W., Behrens, T.E. & Wallis, J.D. Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat. Neurosci. 14, 1581–1589 (2011).
Abe, H. & Lee, D. Distributed coding of actual and hypothetical outcomes in the orbital and dorsolateral prefrontal cortex. Neuron 70, 731–741 (2011).
Tsujimoto, S., Genovesio, A. & Wise, S.P. Monkey orbitofrontal cortex encodes response choices near feedback time. J. Neurosci. 29, 2569–2574 (2009).
Schultz, W., Dayan, P. & Montague, P.R. A neural substrate of prediction and reward. Science 275, 1593–1599 (1997).
Sato, M. & Hikosaka, O. Role of primate substantia nigra pars reticulata in reward-oriented saccadic eye movement. J. Neurosci. 22, 2363–2373 (2002).
Shidara, M., Aigner, T.G. & Richmond, B.J. Neuronal signals in the monkey ventral striatum related to progress through a predictable series of trials. J. Neurosci. 18, 2613–2625 (1998).
Santos, G.S., Nagasaka, Y., Fujii, N. & Nakahara, H. Encoding of social state information by neuronal activities in the macaque caudate nucleus. Soc. Neurosci. 7, 42–58 (2012).
Matsumoto, M. & Hikosaka, O. Representation of negative motivational value in the primate lateral habenula. Nat. Neurosci. 12, 77–84 (2009).
Paton, J.J., Belova, M.A., Morrison, S.E. & Salzman, C.D. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439, 865–870 (2006).
Moll, J. et al. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc. Natl. Acad. Sci. USA 103, 15623–15628 (2006).
Harbaugh, W.T., Mayr, U. & Burghart, D.R. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science 316, 1622–1625 (2007).
Hare, T.A., Camerer, C.F., Knoepfle, D.T. & Rangel, A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J. Neurosci. 30, 583–590 (2010).
Izuma, K., Saito, D.N. & Sadato, N. Processing of the incentive for social approval in the ventral striatum during charitable donation. J. Cogn. Neurosci. 22, 621–631 (2010).
Kuss, K. et al. A reward prediction error for charitable donations reveals outcome orientation of donators. Soc. Cogn. Affect. Neurosci. published online, doi:10.1093/scan/nsr088 (23 December 2011).
Gold, J.I. & Shadlen, M.N. The neural basis of decision making. Annu. Rev. Neurosci. 30, 535–574 (2007).
Sohn, J.W. & Lee, D. Effects of reward expectancy on sequential eye movements in monkeys. Neural Netw. 19, 1181–1191 (2006).
Bowman, E.M., Aigner, T.G. & Richmond, B.J. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine rewards. J. Neurophysiol. 75, 1061–1073 (1996).
O'Doherty, J. et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304, 452–454 (2004).
Chang, S.W., Winecoff, A.A. & Platt, M.L. Vicarious reinforcement in rhesus macaques (Macaca mulatta). Front. Neurosci. 5, 27 (2011).
Chang, S.W., Barter, J.W., Ebitz, R.B., Watson, K.K. & Platt, M.L. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proc. Natl. Acad. Sci. USA 109, 959–964 (2012).
Carter, C.S. et al. Anterior cingulate cortex, error detection and the online monitoring of performance. Science 280, 747–749 (1998).
Ito, S., Stuphorn, V., Brown, J.W. & Schall, J.D. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science 302, 120–122 (2003).
Alexander, W.H. & Brown, J.W. Medial prefrontal cortex as an action-outcome predictor. Nat. Neurosci. 14, 1338–1344 (2011).
Amodio, D.M. & Frith, C.D. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277 (2006).
Azzi, J.C., Sirigu, A. & Duhamel, J.R. Modulation of value representation by social context in the primate orbitofrontal cortex. Proc. Natl. Acad. Sci. USA 109, 2126–2131 (2012).
Cohen, Y.E. & Andersen, R.A. A common reference frame for movement plans in the posterior parietal cortex. Nat. Rev. Neurosci. 3, 553–562 (2002).
Yoshida, K., Saito, N., Iriki, A. & Isoda, M. Representation of others' action by neurons in monkey medial frontal cortex. Curr. Biol. 21, 249–253 (2011).
Saxe, R. Uniquely human social cognition. Curr. Opin. Neurobiol. 16, 235–239 (2006).
Waytz, A., Zaki, J. & Mitchell, J.P. Response of dorsomedial prefrontal cortex predicts altruistic behavior. J. Neurosci. 32, 7646–7650 (2012).
Mobbs, D. et al. A key role for similarity in vicarious reward. Science 324, 900 (2009).
Seo, H. & Lee, D. Cortical mechanisms for reinforcement learning in competitive games. Phil. Trans. R. Soc. Lond. B 363, 3845–3857 (2008).
Burke, C.J., Tobler, P.N., Baddeley, M. & Schultz, W. Neural mechanisms of observational learning. Proc. Natl. Acad. Sci. USA 107, 14431–14436 (2010).
Hampton, A.N., Bossaerts, P. & O'Doherty, J.P. Neural correlates of mentalizing-related computations during strategic interactions in humans. Proc. Natl. Acad. Sci. USA 105, 6741–6746 (2008).
Jeon, D. et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci. 13, 482–488 (2010).
Singer, T. et al. Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157–1162 (2004).
Paxinos, G., Huang, X.F & Toga, A.W. The Rhesus Monkey Brain in Stereotaxic Coordinates (Academic Press, 2000).
Vogt, B.A. & Pandya, D.N. Cingulate cortex of the rhesus monkey. II. Cortical afferents. J. Comp. Neurol. 262, 271–289 (1987).
Carmichael, S.T. & Price, J.L. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 363, 615–641 (1995).
Acknowledgements
We thank J.M. Groh, J.M. Pearson, D.L. Barack, R.B. Ebitz, E.S. Bromberg-Martin, K.K. Watson and B.Y. Hayden for helpful discussions. We are very grateful to S.P. Wise and C. Padoa-Schioppa for insightful discussions and comments on earlier versions of the manuscript. We also thank M.L. Carlson for general technical assistance. This work was supported by a T32 Postdoctoral Training Grant on Fundamental and Translational Neuroscience (S.W.C.C., 2T32NS051156–06), a Ruth K. Broad Biomedical Foundation Postdoctoral Grant (S.W.C.C.), a Canadian Institutes of Health Research Doctoral research award (J.-F.G., 84765), the National Institute of Mental Health (M.L.P. and S.W.C.C., MH095894) and the Department of Defense (M.L.P. and S.W.C.C., W81XWH-11-1-0584).
Author information
Authors and Affiliations
Contributions
S.W.C.C. and M.L.P. designed the study and wrote the paper. S.W.C.C. and J.-F.G. performed the experiments and S.W.C.C. analyzed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Table 1 (PDF 662 kb)
Rights and permissions
About this article
Cite this article
Chang, S., Gariépy, JF. & Platt, M. Neuronal reference frames for social decisions in primate frontal cortex. Nat Neurosci 16, 243–250 (2013). https://doi.org/10.1038/nn.3287
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3287
This article is cited by
-
Visuo-frontal interactions during social learning in freely moving macaques
Nature (2024)
-
Neural signatures of natural behaviour in socializing macaques
Nature (2024)
-
L-DOPA and oxytocin influence the neural correlates of performance monitoring for self and others
Psychopharmacology (2024)
-
Social circuits and their dysfunction in autism spectrum disorder
Molecular Psychiatry (2023)
-
Unselfish traits and social decision-making patterns characterize six populations of real-world extraordinary altruists
Nature Communications (2023)