Abstract
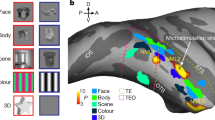
Visual-object processing culminates in inferior temporal cortex (IT). To assess the organization of IT, we measured functional magnetic resonance imaging responses in alert monkeys to achromatic images (faces, fruit, bodies and places) and colored gratings. IT contained multiple color-biased regions, which were typically ventral to face patches and yoked to them, spaced regularly at four locations predicted by known anatomy. Color and face selectivity increased for more anterior regions, indicative of a broad hierarchical arrangement. Responses to non-face shapes were found across IT, but were stronger outside color-biased regions and face patches, consistent with multiple parallel streams. IT also contained multiple coarse eccentricity maps: face patches overlapped central representations, color-biased regions spanned mid-peripheral representations and place-biased regions overlapped peripheral representations. These results show that IT comprises parallel, multi-stage processing networks subject to one organizing principle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
DiCarlo, J.J., Zoccolan, D. & Rust, N.C. How does the brain solve visual object recognition? Neuron 73, 415–434 (2012).
Tsao, D.Y.F.W., Knutsen, T.A., Mandeville, J.B. & Tootell, R.B.H. Faces and objects in macaque cerebral cortex. Nat. Neurosci. 6, 989–995 (2003).
Pinsk, M.A., DeSimone, K., Moore, T., Gross, C.G. & Kastner, S. Representations of faces and body parts in macaque temporal cortex: a functional MRI study. Proc. Natl. Acad. Sci. USA 102, 6996–7001 (2005).
Ku, S.P., Tolias, A.S., Logothetis, N.K. & Goense, J. fMRI of the face-processing network in the ventral temporal lobe of awake and anesthetized macaques. Neuron 70, 352–362 (2011).
Freiwald, W.A. & Tsao, D.Y. Functional compartmentalization and viewpoint generalization within the macaque face-processing system. Science 330, 845–851 (2010).
Srihasam, K., Mandeville, J.B., Morocz, I.A., Sullivan, K.J. & Livingstone, M.S. Behavioral and anatomical consequences of early versus late symbol training in macaques. Neuron 73, 608–619 (2012).
Op de Beeck, H.P., Deutsch, J.A., Vanduffel, W., Kanwisher, N.G. & DiCarlo, J.J. A stable topography of selectivity for unfamiliar shape classes in monkey inferior temporal cortex. Cereb. Cortex 18, 1676–1694 (2008).
Bell, A.H., Hadj-Bouziane, F., Frihauf, J.B., Tootell, R.B. & Ungerleider, L.G. Object representations in the temporal cortex of monkeys and humans as revealed by functional magnetic resonance imaging. J. Neurophysiol. 101, 688–700 (2009).
Cox, D., Meyers, E. & Sinha, P. Contextually evoked object-specific responses in human visual cortex. Science 304, 115–117 (2004).
Stoughton, C.M., Lafer-Sousa, R., Gagin, G. & Conway, B.R. Psychophysical chromatic mechanisms in macaque monkey. J. Neurosci. 32, 15216–15226 (2012).
Tootell, R.B., Nelissen, K., Vanduffel, W. & Orban, G.A. Search for color 'center(s)' in macaque visual cortex. Cereb. Cortex 14, 353–363 (2004).
Conway, B.R. & Tsao, D.Y. Color architecture in alert macaque cortex revealed by fMRI. Cereb. Cortex 16, 1604–1613 (2006).
Conway, B.R., Moeller, S. & Tsao, D.Y. Specialized color modules in macaque extrastriate cortex. Neuron 56, 560–573 (2007).
Harada, T. et al. Distribution of colour-selective activity in the monkey inferior temporal cortex revealed by functional magnetic resonance imaging. Eur. J. Neurosci. 30, 1960–1970 (2009).
Katsuyama, N. et al. Cortical activation during color discrimination task in macaques as revealed by positron emission tomography. Neurosci. Lett. 484, 168–173 (2010).
Iwai, E. & Yukie, M. Amygdalofugal and amygdalopetal connections with modality-specific visual cortical areas in macaques (Macaca fuscata, M. mulatta and M. fascicularis). J. Comp. Neurol. 261, 362–387 (1987).
Van Essen, D.C., Felleman, D.J., DeYoe, E.A., Olavarria, J. & Knierim, J. Modular and hierarchical organization of extrastriate visual cortex in the macaque monkey. Cold Spring Harb. Symp. Quant. Biol. 55, 679–696 (1990).
Kravitz, D.J., Saleem, K.S., Baker, C.I., Ungerleider, L.G. & Mishkin, M. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn. Sci. 17, 26–49 (2013).
Grill-Spector, K. & Malach, R. The human visual cortex. Annu. Rev. Neurosci. 27, 649–677 (2004).
Kanwisher, N. Functional specificity in the human brain: a window into the functional architecture of the mind. Proc. Natl. Acad. Sci. USA 107, 11163–11170 (2010).
Hadjikhani, N., Liu, A.K., Dale, A.M., Cavanagh, P. & Tootell, R.B. Retinotopy and color sensitivity in human visual cortical area V8. Nat. Neurosci. [see comments] 1, 235–241 (1998).
Bartels, A. & Zeki, S. The architecture of the colour centre in the human visual brain: new results and a review. Eur. J. Neurosci. 12, 172–193 (2000).
Hasson, U., Harel, M., Levy, I. & Malach, R. Large-scale mirror-symmetry organization of human occipito-temporal object areas. Neuron 37, 1027–1041 (2003).
Boussaoud, D., Desimone, R. & Ungerleider, L.G. Visual topography of area TEO in the macaque. J. Comp. Neurol. 306, 554–575 (1991).
Yasuda, M., Banno, T. & Komatsu, H. Color selectivity of neurons in the posterior inferior temporal cortex of the macaque monkey. Cereb. Cortex 20, 1630–1646 (2010).
Derrington, A.M., Krauskopf, J. & Lennie, P. Chromatic mechanisms in lateral geniculate nucleus of macaque. J. Physiol. (Lond.) 357, 241–265 (1984).
Koida, K. & Komatsu, H. Effects of task demands on the responses of color-selective neurons in the inferior temporal cortex. Nat. Neurosci. 10, 108–116 (2007).
Matsumora, T., Koida, K. & Komatsu, H. Relationship between color discrimination and neural responses in the inferior temporal cortex of the monkey. J. Neurophysiol. 100, 3361–3374 (2008).
Stoughton, C.M. & Conway, B.R. Neural basis for unique hues. Curr. Biol. 18, R698–R699 (2008).
Conway, B.R. & Tsao, D.Y. Color-tuned neurons are spatially clustered according to color preference within alert macaque posterior inferior temporal cortex. Proc. Natl. Acad. Sci. USA 106, 18034–18039 (2009).
Tsao, D.Y., Moeller, S. & Freiwald, W.A. Comparing face patch systems in macaques and humans. Proc. Natl. Acad. Sci. USA 105, 19514–19519 (2008).
Cavanagh, P. & Leclerc, Y.G. Shape from shadows. J. Exp. Psychol. Hum. Percept. Perform. 15, 3–27 (1989).
Rolls, E.T. Face processing in different brain areas, and critical band masking. J. Neuropsychol. 2, 325–360 (2008).
Van Essen, D.C. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385, 313–318 (1997).
Conover, W.J. Practical Nonparametric Statistics, 2nd edn. (John Wiley & Sons, New York, 1980).
Karnath, H.O. New insights into the functions of the superior temporal cortex. Nat. Rev. Neurosci. 2, 568–576 (2001).
Baker, C.I. et al. Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proc. Natl. Acad. Sci. USA 104, 9087–9092 (2007).
Tanaka, K., Saito, H., Fukada, Y. & Moriya, M. Coding visual images of objects in the inferotemporal cortex of the macaque monkey. J. Neurophysiol. 66, 170–189 (1991).
Kemp, R., Pike, G., White, P. & Musselman, A. Perception and recognition of normal and negative faces: the role of shape from shading and pigmentation cues. Perception 25, 37–52 (1996).
Moro, V. et al. The neural basis of body form and body action agnosia. Neuron 60, 235–246 (2008).
Conway, B.R. et al. Advances in color science: from retina to behavior. J. Neurosci. 30, 14955–14963 (2010).
Iwai, E. & Yukie, M. A direct projection from hippocampal field CA1 to ventral area TE of inferotemporal cortex in the monkey. Brain Res. 444, 397–401 (1988).
Konkle, T. & Oliva, A. A real-world size organization of object responses in occipitotemporal cortex. Neuron 74, 1114–1124 (2012).
Allman, J.M. & Kaas, J.H. A crescent-shaped cortical visual area surrounding the middle temporal area (MT) in the owl monkey (Aotus trivirgatus). Brain Res. 81, 199–213 (1974).
Sereno, M.l. & Allman, J.M. Cortical visual areas in mammals. in A. G. Leventhal (ed.) The Neural Basis of Visual Function (ed. Leventhal, A.G.) 160–172 (Macmillan, London, 1991).
Zeki, S. The representation of colours in the cerebral cortex. Nature 284, 412–418 (1980).
Roe, A.W. et al. Towards a unified theory of visual area V4. Neuron 74, 12–29 (2012).
Heywood, C.A. & Kentridge, R.W. Achromatopsia, color vision, and cortex. Neurol. Clin. 21, 483–500 (2003).
Bouvier, S.E. & Engel, S.A. Behavioral deficits and cortical damage loci in cerebral achromatopsia. Cereb. Cortex 16, 183–191 (2006).
Kimmlingen, R. et al. An easy to exchange high performance head gradient insert for a 3T whole body MRI system: first results. Proc. Intl. Soc. Magn. Reson. Med. Sci. Meet. Exhib. 11, 1630 (2004).
Hansen, T., Giesel, M. & Gegenfurtner, K.R. Chromatic discrimination of natural objects. J. Vis. 8, 2 1–19 (2008).
Smith, V.C. & Pokorny, J. Spectral sensitivity of color-blind observers and the cone photopigments. Vision Res. 12, 2059–2071 (1972).
Johnson, E.N., Hawken, M.J. & Shapley, R. Cone inputs in macaque primary visual cortex. J. Neurophysiol. 91, 2501–2514 (2004).
Fize, D. et al. The retinotopic organization of primate dorsal V4 and surrounding areas: a functional magnetic resonance imaging study in awake monkeys. J. Neurosci. 23, 7395–7406 (2003).
Lafer-Sousa, R., Liu, Y.O., Lafer-Sousa, L., Wiest, M.C. & Conway, B.R. Color tuning in alert macaque V1 assessed with fMRI and single-unit recording shows a bias toward daylight colors. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 29, 657–670 (2012).
Paxinos, G., Huang, X.-F. & Toga, A.W. The Rhesus Monkey Brain in Stereotaxic Coordinates (Academic Press, San Diego, 2000).
Cox, R.W. & Hyde, J.S. Software tools for analysis and visualization of fMRI data. NMR Biomed. 10, 171–178 (1997).
Kriegeskorte, N., Simmons, W.K., Bellgowan, P.S. & Baker, C.I. Circular analysis in systems neuroscience: the dangers of double dipping. Nat. Neurosci. 12, 535–540 (2009).
Simmons, W.K., Bellgowan, P.S. & Martin, A. Measuring selectivity in fMRI data. Nat. Neurosci. 10, 4–5 (2007).
Baker, C.I., Hutchison, T.L. & Kanwisher, N. Does the fusiform face area contain subregions highly selective for nonfaces? Nat. Neurosci. 10, 3–4 (2007).
Acknowledgements
fMRI data were analyzed using the jip toolbox generously provided by J. Mandeville (Massachusetts General Hospital, http://www.nitrc.org/projects/jip/), and analysis scripts compiled by S. Moeller (RWTH Aachen University). We thank J. Maunsell, D. Hubel and M. Livingstone for research space, helpful discussions and assistance with animal protocols. A. Rehding, N. Kanwisher, J. DiCarlo and P. Mayo provided valuable comments on the manuscript. B.R.C. gratefully acknowledges seminal conversations that took place with D. Tsao, with whom the idea to map multiple stimulus dimensions in IT was conceived and initially begun. We thank Y. Liu for implementing the simulation shown in Figure 5 and help with MRI analysis. We thank J. McCready and E. Erzinger, who designed and built the fMRI primate chair, and C. Stoughton and G. Gagin, who trained the animals. We thank L. Wald and A. Mareyam for providing the four-channel magnetic resonance coil. We thank W. Vanduffel, J. Mandeville, J. Polimeni, T. Witzel, M. O'Hara, G. Khandewal, M. Histed and P. Mayo for help running the experiments and conducting the analyses. This paper is dedicated to the memory of our mentor and beloved friend, David Hubel, who died September 22, 2013 (age 87). This work was supported by grants from the National Science Foundation (0918064), the US National Institutes of Health (EY023322), the Whitehall Foundation, the Radcliffe Institute for Advanced Study (Harvard University) and Wellesley College. This research was carried out at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies (P41EB015896) and a P41 Biotechnology Resource grant supported by the National Institute of Biomedical Imaging and Bioengineering. This work also involved the use of instrumentation supported by the US National Institutes of Health Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program (S10RR021110).
Author information
Authors and Affiliations
Contributions
R.L.-S. carried out the experiments and analyzed the data. B.R.C. designed the research, carried out the experiments, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 a. Comparison of the responses during meridian mapping using BOLD and a contrast agent (MION).
%Signal change (response to vertical meridian stimulation minus response to horizontal meridian stimulation; see Figure 1) was assessed in two regions of interest, the V1-V2 vertical meridian representation and the PIT/CIT vertical meridian representation. MION substantially enhanced the signal. Data were obtained in the same animal and the BOLD and MION experiments comprises the same number of stimulus runs (N=8). b, Plot showing the C.I.E. xy coordinates of the color stimuli used (left) and the C.I.E. uv coordinates (right).The cardinal axes of DKL color space are shown: L-M and S. C.I.E. Luv space is approximately perceptually uniform. The center point indicates the adaptation point.
Supplementary Figure 2 a, Activation maps showing responses to color versus responses to black-and-white using colors 7 & 8 (a) and colors 1-6 and 9-12 (b) in M1 (left) and M2 (right).
The bottom row in (a) and b shows the 20° ventral rotated view corresponding to Figure 3a. Colors 7 and 8 are the two colors least likely to be contaminated by luminance artifacts (see text). (c) Color-biased regions identified by comparing responses to all colors besides colors 7 & 8 versus responses to achromatic gratings, using no spatial smoothing, in M1. Bottom set of panels shows expanded view of early retinotopic cortex, showing comparable pattern of activation to that described by Conway et al. (2007). Note the luminance bias within V3, and the suggestion of a stripe pattern within V2, presumably reflecting the concentration of color cells within the cytochrome-oxidase thin stripes. Unlike the earlier report, color stimuli were calibrated using the DKL color space, and the saturation of the achromatic gratings was adjusted so that V1 gave the same magnitude of response to the achromatic gratings as it gave to colors 7&8. This calibration procedure was effective, as shown by the lack of a color bias in V1.
Supplementary Figure 3 (a) Time course of the responses to colors within color-biased regions (top) and face-biased regions (bottom) of posterior, central, anterior and anterior-medial IT within the two monkeys (M1, M2).
(b) Time course of the responses to images of objects within color-biased regions (top) and face-biased regions (bottom) of posterior, central, anterior and anterior-medial IT within the two monkeys (M1,M2).
Supplementary Figure 4 Face-biased activation within the hippocampus, anterior insula, entorhinal cortex and amygdala in M1.
Right panels show time courses of the responses to frontal unfamiliar faces (f), bodies (b), familiar faces (ff, cage mate), vegetables & fruits (vf), familiar faces (fs, self), and places (p). Slices show Talairach coordinates.
Supplementary Figure 5 Raw functional echo planar (EPI) coronal images showing MION activation every 1 mm; color-biased activation (blue-cyan, left panels) and face-biased activation (orange-red, right panels) of M2.
Table below shows the approximate Talairach coordinates of face patch and color-biased region centers for M1 and M2.
Supplementary Figure 6 Activation patterns for one monkey (M1), both hemispheres, generated by various contrasts, as indicated.
Black contours show face patches; white contours show color-biased regions. Inset in box shows the activation pattern to all non-face objects compared to scrambled objects.
Supplementary Figure 7 Eccentricity mapping.
a, Spatial distribution of the responses to the eccentricity stimulus within both hemispheres (left hemisphere, top; right hemisphere, bottom) of M2. The left panel of each row shows in blue-cyan the responses that were greater to the foveal disc (1.5°) than to the other eccentricity stimuli combined. In the response maps, the spot of orange-red within the annulus of cyan corresponds to the fixation spot. The subsequent panels show responses to progressively expanding annuli (the radii of the annuli is given). The stimulus comprised a checkerboard of colors defined by the L-M axis and one defined by the S-axis. Note that progressively expanding annuli elicit correspondingly wider annuli of activation within the cortex, as shown by others (e.g. Fize et al, 2003). In addition, along IT is evident clear activation to the central disc and smallest annulus. b, the left panel shows the activation pattern comparing the first two eccentricity conditions with the last two (and is what is reported in Figure 6). For comparison, the right panel shows the results of the meridian mapping experiment. Along IT is shown higher activation to stimulation along the vertical meridian at locations in between the central visual field representations. We do not interpret these as vertical meridian representations, but rather as indication of a peripheral bias: the vertical meridian stimulus comprised 60° wedges, while the horizontal meridian stimulus was 30°. Thus on average, the vertical stimulus represented more activation of the peripheral visual field.
Supplementary Figure 8 Bar plots quantifying the responses to central and peripheral stimulation within color-biased regions (a) and face patches (b) along the posterior-anterior axis.
Compare with Figure 6c, showing the responses within a single combined color-biased region of interest and single combined face-biased region of interest. Error bars indicate s.e. (N=4 hemispheres). c, Histograms of distances from each IT face-selective voxel to its nearest color-selective voxel (red line, mean = 4.0 mm, s.d. 0.2 mm), and from each IT face-selective voxel to its nearest place-selective voxel (blue line, mean = 5.3 mm). The histograms were normalized by the total number of comparisons made. On average, the distance between face-biased and color-biased voxels is shorter than the distance between face-biased voxels and place-biased voxels (Mann–Whitney–Wilcoxon test, p= 1.5x10-24).
Supplementary Figure 9 Extent of visually active voxels in M1.
(a) Activation is found along the full extent of the temporal lobe. (b) time course of response to color 8 and the achromatic gratings within the IT color regions, the IT regions more significantly responsive to achromatic gratings (IT BW), the entire IT volume, and the portions of IT outside of color-biased and luminance-biased regions. Color-biased regions are shown with white contours and face patches are shown with black contours. The patchiness of these functionally biased regions cannot be attributed to lack of signal within the intervening tissue. (c) Color-biased (top row) and face-biased (bottom row) regions shown at three significance level thresholds in M1, right hemisphere.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 3856 kb)
Rights and permissions
About this article
Cite this article
Lafer-Sousa, R., Conway, B. Parallel, multi-stage processing of colors, faces and shapes in macaque inferior temporal cortex. Nat Neurosci 16, 1870–1878 (2013). https://doi.org/10.1038/nn.3555
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3555
This article is cited by
-
Brain Activity is Influenced by How High Dimensional Data are Represented: An EEG Study of Scatterplot Diagnostic (Scagnostics) Measures
Journal of Healthcare Informatics Research (2024)
-
High-dimensional topographic organization of visual features in the primate temporal lobe
Nature Communications (2023)
-
A domain-relevant framework for the development of face processing
Nature Reviews Psychology (2023)
-
Perception of Color and Its Encoding in the Cortex in Primates
Neuroscience and Behavioral Physiology (2023)
-
A novel and smarter model to authenticate and identify people intelligently for security purposes
Telecommunication Systems (2023)