Abstract
In the reward circuitry of the brain, α-7-nicotinic acetylcholine receptors (α7nAChRs) modulate effects of Δ9-tetrahydrocannabinol (THC), marijuana's main psychoactive ingredient. Kynurenic acid (KYNA) is an endogenous negative allosteric modulator of α7nAChRs. Here we report that the kynurenine 3-monooxygenase (KMO) inhibitor Ro 61-8048 increases brain KYNA levels and attenuates cannabinoid-induced increases in extracellular dopamine in reward-related brain areas. In the self-administration model of drug abuse, Ro 61-8048 reduced the rewarding effects of THC and the synthetic cannabinoid WIN 55,212-2 in squirrel monkeys and rats, respectively, and it also prevented relapse to drug-seeking induced by reexposure to cannabinoids or cannabinoid-associated cues. The effects of enhancing endogenous KYNA levels with Ro 61-8048 were prevented by positive allosteric modulators of α7nAChRs. Despite a clear need, there are no medications approved for treatment of marijuana dependence. Modulation of KYNA offers a pharmacological strategy for achieving abstinence from marijuana and preventing relapse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings (Rockville, Maryland, USA, 2010).
Gardner, E.L. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol. Biochem. Behav. 81, 263–284 (2005).
Solinas, M., Goldberg, S.R. & Piomelli, D. The endocannabinoid system in brain reward processes. Br. J. Pharmacol. 154, 369–383 (2008).
Diana, M., Melis, M. & Gessa, G.L. Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur. J. Neurosci. 10, 2825–2830 (1998).
French, E.D., Dillon, K. & Wu, X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport 8, 649–652 (1997).
Chen, J.P. et al. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl.) 102, 156–162 (1990).
Tanda, G., Pontieri, F.E. & Di Chiara, G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276, 2048–2050 (1997).
Dani, J.A. & Bertrand, D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 47, 699–729 (2007).
Fu, Y., Matta, S.G., Gao, W., Brower, V.G. & Sharp, B.M. Systemic nicotine stimulates dopamine release in nucleus accumbens: re-evaluation of the role of N-methyl-D-aspartate receptors in the ventral tegmental area. J. Pharmacol. Exp. Ther. 294, 458–465 (2000).
Kaiser, S. & Wonnacott, S. alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol. Pharmacol. 58, 312–318 (2000).
Solinas, M. et al. Nicotinic alpha 7 receptors as a new target for treatment of cannabis abuse. J. Neurosci. 27, 5615–5620 (2007).
Weinstein, A.M. & Gorelick, D.A. Pharmacological treatment of cannabis dependence. Curr. Pharm. Des. 17, 1351–1358 (2011).
Arias, H.R. et al. Role of non-neuronal nicotinic acetylcholine receptors in angiogenesis. Int. J. Biochem. Cell Biol. 41, 1441–1451 (2009).
Roegge, C.S. & Levin, E.D. Nicotinic receptor antagonists in rats. in Animal Models of Cognitive Impairment (eds. Levin, E.D. & Buccafusco, J.J.) (CRC, Boca Raton, Florida, USA, 2006).
Arias, H.R. Positive and negative modulation of nicotinic receptors. Adv. Protein Chem. Struct. Biol. 80, 153–203 (2010).
Bertrand, D. et al. Positive allosteric modulation of the alpha7 nicotinic acetylcholine receptor: ligand interactions with distinct binding sites and evidence for a prominent role of the M2–M3 segment. Mol. Pharmacol. 74, 1407–1416 (2008).
Christopoulos, A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat. Rev. Drug Discov. 1, 198–210 (2002).
Perkins, M.N. & Stone, T.W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 247, 184–187 (1982).
Kiss, C. et al. Kynurenate production by cultured human astrocytes. J. Neural Transm. 110, 1–14 (2003).
Moroni, F., Russi, P., Lombardi, G., Beni, M. & Carla, V. Presence of kynurenic acid in the mammalian brain. J. Neurochem. 51, 177–180 (1988).
Kessler, M., Terramani, T., Lynch, G. & Baudry, M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J. Neurochem. 52, 1319–1328 (1989).
Alkondon, M. et al. Targeted deletion of the kynurenine aminotransferase ii gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via alpha7 nicotinic receptors in the hippocampus. J. Neurosci. 24, 4635–4648 (2004).
Hilmas, C. et al. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J. Neurosci. 21, 7463–7473 (2001).
Lopes, C. et al. Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at alpha7* nicotinic receptors. J. Pharmacol. Exp. Ther. 322, 48–58 (2007).
Amori, L. et al. Specific inhibition of kynurenate synthesis enhances extracellular dopamine levels in the rodent striatum. Neuroscience 159, 196–203 (2009).
Wu, H.Q. et al. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J. Mol. Neurosci. 40, 204–210 (2010).
Zmarowski, A. et al. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur. J. Neurosci. 29, 529–538 (2009).
Carpenedo, R. et al. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur. J. Neurosci. 13, 2141–2147 (2001).
Rassoulpour, A., Wu, H.Q., Ferre, S. & Schwarcz, R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J. Neurochem. 93, 762–765 (2005).
Schwarcz, R., Bruno, J.P., Muchowski, P.J. & Wu, H.Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477 (2012).
Justinova, Z., Tanda, G., Redhi, G.H. & Goldberg, S.R. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl.) 169, 135–140 (2003).
Justinova, Z. et al. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol. Psychiatry 64, 930–937 (2008).
Amaral, M. et al. Structural basis of kynurenine 3-monooxygenase inhibition. Nature 496, 382–385 (2013).
Moroni, F. Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Eur. J. Pharmacol. 375, 87–100 (1999).
Grégoire, L. et al. Prolonged kynurenine 3-hydroxylase inhibition reduces development of levodopa-induced dyskinesias in parkinsonian monkeys. Behav. Brain Res. 186, 161–167 (2008).
Zwilling, D. et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell 145, 863–874 (2011).
Fukui, S., Schwarcz, R., Rapoport, S.I., Takada, Y. & Smith, Q.R. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J. Neurochem. 56, 2007–2017 (1991).
Turski, W.A., Gramsbergen, J.B., Traitler, H. & Schwarcz, R. Rat brain slices produce and liberate kynurenic acid upon exposure to L-kynurenine. J. Neurochem. 52, 1629–1636 (1989).
Turski, W.A. & Schwarcz, R. On the disposition of intrahippocampally injected kynurenic acid in the rat. Exp. Brain Res. 71, 563–567 (1988).
Uwai, Y., Honjo, H. & Iwamoto, K. Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol. Res. 65, 254–260 (2012).
Röver, S., Cesura, A.M., Huguenin, P., Kettler, R. & Szente, A. Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J. Med. Chem. 40, 4378–4385 (1997).
Hurst, R.S. et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J. Neurosci. 25, 4396–4405 (2005).
Chess, A.C., Simoni, M.K., Alling, T.E. & Bucci, D.J. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr. Bull. 33, 797–804 (2007).
Pocivavsek, A. et al. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology 36, 2357–2367 (2011).
Navarrete, M. & Araque, A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126 (2010).
Pistis, M., Porcu, G., Melis, M., Diana, M. & Gessa, G.L. Effects of cannabinoids on prefrontal neuronal responses to ventral tegmental area stimulation. Eur. J. Neurosci. 14, 96–102 (2001).
Pistis, M., Muntoni, A.L., Pillolla, G. & Gessa, G.L. Cannabinoids inhibit excitatory inputs to neurons in the shell of the nucleus accumbens: an in vivo electrophysiological study. Eur. J. Neurosci. 15, 1795–1802 (2002).
Panlilio, L.V. et al. Combined effects of THC and caffeine on working memory in rats. Br. J. Pharmacol. 165, 2529–2538 (2012).
Le Foll, B., Gorelick, D.A. & Goldberg, S.R. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology (Berl.) 205, 171–174 (2009).
Cozzi, A., Carpenedo, R. & Moroni, F. Kynurenine hydroxylase inhibitors reduce ischemic brain damage: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3,4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2yl]-benzenesulfonamide (Ro 61–8048) in models of focal or global brain ischemia. J. Cereb. Blood Flow Metab. 19, 771–777 (1999).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates (Academic, San Diego, 1998).
Fadda, P., Scherma, M., Fresu, A., Collu, M. & Fratta, W. Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse 50, 1–6 (2003).
Fattore, L. et al. Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2. Eur. J. Neurosci. 25, 2191–2200 (2007).
Goldberg, S.R. Comparable behavior maintained under fixed-ratio and second-order schedules of food presentation, cocaine injection or d-amphetamine injection in the squirrel monkey. J. Pharmacol. Exp. Ther. 186, 18–30 (1973).
Panlilio, L.V., Yasar, S., Thorndike, E.B., Goldberg, S.R. & Schindler, C.W. Automatic recording of mediating behavior in delayed matching- and nonmatching-to-position procedures in rats. Psychopharmacology (Berl.) 214, 495–504 (2011).
Kangas, B.D., Berry, M.S. & Branch, M.N. On the development and mechanics of delayed matching-to-sample performance. J. Exp. Anal. Behav. 95, 221–236 (2011).
Kangas, B.D. & Bergman, J. A novel touch-sensitive apparatus for behavioral studies in unrestrained squirrel monkeys. J. Neurosci. Methods 209, 331–336 (2012).
Solinas, M., Panlilio, L.V., Antoniou, K., Pappas, L.A. & Goldberg, S.R. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl) -4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J. Pharmacol. Exp. Ther. 306, 93–102 (2003).
Kangas, B.D. et al. Cannabinoid discrimination and antagonism by CB(1) neutral and inverse agonist antagonists. J. Pharmacol. Exp. Ther. 344, 561–567 (2013).
Acknowledgements
We thank E. Thorndike for excellent technical assistance during the rodent studies. We thank I. Baum, S. Stevens and P. White for excellent veterinary assistance during the primate studies. This study was supported in part by the Intramural Research Program of the National Institute on Drug Abuse (NIDA), National Institutes of Health, Department of Health and Human Services, NIDA Residential Research Support Services Contract N01 DA59909 (principal investigator D. Kelly), by the Italian Ministry of University and Scientific Research, by DA023142 (J.B.) and DA035974 (B.D.K.) and by the Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine. T.Z. was supported by a Grant from Regione Autonoma della Sardegna and by the European Social Fund, LR7 2007.
Author information
Authors and Affiliations
Contributions
Z.J. was involved in the design of the study, supervised and analyzed the primate self-administration and reinstatement experiments and rodent drug discrimination experiments, and wrote the first draft of the manuscript. P.M. was involved in design of the study, conducted microdialysis experiments including collection of all samples, performed dopamine assay, analyzed microdialysis data and helped prepare the final draft of the manuscript. H.-Q.W. analyzed KYNA levels in microdialysis samples. M.E.S. conducted and analyzed microdialysis experiments with local KYNA infusion and PNU120596. G.H.R. conducted the primate self-administration and reinstatement experiments. L.V.P designed and analyzed the experiments with delayed nonmatching to position and was involved in discussions of the data. C.B. conducted rodent drug discrimination experiments and was involved in discussions of the data. T.Z. conducted the rat self-administration and microdialysis experiments with WIN 55,212-2. M. Scherma supervised the rat self-administration and microdialysis experiments with WIN 55,212-2, analyzed the data and was involved in the discussions of the data. W.F. was involved in the discussions of the data. A.P. assisted in conducting the microdialysis experiments with THC. J.B. and B.D.K. designed, conducted and analyzed the data from the primate discrimination and memory experiments. S.F., M. Solinas, M.P. and G.T. were involved in the design of the study and discussions of the data. R.S. and S.R.G. conceived, designed and supervised the study and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
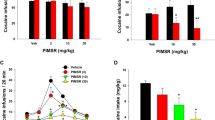
Supplementary Figure 1 Ro 61-8048 reverses the depressant effects of THC on food self-administration in monkeys.
THC (0.56 mg/kg, i.v., immediately before the session) significantly decreased the number of food pellets self-administered over one-h sessions (a) and also decreased overall response rates (b) by squirrel monkeys under a fixed-ratio ten (FR10) schedule. Ro 61-8048 (20 mg/kg i.m., 40 min before session) did not significantly affect food-reinforced behavior, but reversed the effects of THC. The number of food pellets per session (a) and overall response rates in the presence of the green light signaling food availability (b) are shown. Each bar represents the mean ± s.e.m (n = 3). **P<0.01, post-hoc vs. vehicles condition; # P<0.05, ## P<0.01, post-hoc vs. vehicle + THC 0.56 condition, Bonferroni test.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 (PDF 70 kb)
Rights and permissions
About this article
Cite this article
Justinova, Z., Mascia, P., Wu, HQ. et al. Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nat Neurosci 16, 1652–1661 (2013). https://doi.org/10.1038/nn.3540
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3540
This article is cited by
-
Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond
Nature Reviews Drug Discovery (2019)
-
Astrocytic Mechanisms Involving Kynurenic Acid Control Δ9-Tetrahydrocannabinol-Induced Increases in Glutamate Release in Brain Reward-Processing Areas
Molecular Neurobiology (2019)
-
Preclinical Studies of Cannabinoid Reward, Treatments for Cannabis Use Disorder, and Addiction-Related Effects of Cannabinoid Exposure
Neuropsychopharmacology (2018)
-
Effects of Adolescent Cannabinoid Self-Administration in Rats on Addiction-Related Behaviors and Working Memory
Neuropsychopharmacology (2017)
-
Attenuating Nicotine Reinforcement and Relapse by Enhancing Endogenous Brain Levels of Kynurenic Acid in Rats and Squirrel Monkeys
Neuropsychopharmacology (2017)