Abstract
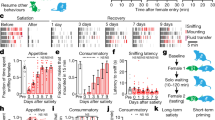
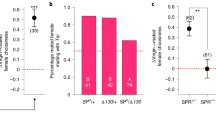
Rival exposure causes Drosophila melanogaster males to prolong mating. Longer mating duration (LMD) may enhance reproductive success, but its underlying mechanism is currently unknown. We found that LMD is context dependent and can be induced solely via visual stimuli. In addition, we found that LMD involves neural circuits that are important for visual memory, including central neurons in the ellipsoid body, but not the mushroom bodies or the fan-shaped bodies, and may rely on the rival exposure memory lasting for several hours. LMD is affected by a subset of learning and memory mutants. LMD depends on the circadian clock genes timeless and period, but not Clock or cycle, and persists in many arrhythmic conditions. Moreover, LMD critically depends on a subset of pigment dispersing factor neurons rather than the entire circadian neural circuit. Our study thus delineates parts of the molecular and cellular basis for LMD, a plastic social behavior elicited by visual cues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Darwin, C. The Descent of Man, and Selection in Relation to Sex (John Murray, 1871).
Kim, Y.-K. Sexual selection and aggressive behavior in Drosophila. in Handbook of Behavior Genetics (ed. Kim, Y.-K.) 317–330 (Springer New York, 2009).
Ridley, M. Seminal work. Nature 397, 576–577 (1999).
Parker, G.A. Sperm competition and its evolutionary consequences in insects. Biol. Rev. Camb. Philos. Soc. 45, 525–567 (1970).
Markow, T.A. Evolution of Drosophila mating systems. Evol. Biol. 29, 73–106 (1996).
Koref-Santibáñez, S. Effects of age and experience on mating activity in the sibling species Drosophila pavani and Drosophila gaucha. Behav. Genet. 31, 287–297 (2001).
Lefranc, A. & Bundgaard, J. The influence of male and female body size on copulation duration and fecundity in Drosophila melanogaster. Hereditas 132, 243–247 (2000).
De Crespigny, F.E.C., Pitt, T.D. & Wedell, N. Increased male mating rate in Drosophila is associated with Wolbachia infection. J. Evol. Biol. 19, 1964–1972 (2006).
Luck, N. & Joly, D. Sexual selection and mating advantages in the giant sperm species, Drosophila bifurca. J. Insect Sci. 5, 10 (2005).
Bretman, A., Fricke, C. & Chapman, T. Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. Proc. Biol. Sci. 276, 1705–1711 (2009).
Mazzi, D., Kesaniemi, J., Hoikkala, A. & Klappert, K. Sexual conflict over the duration of copulation in Drosophila montana: why is longer better? BMC Evol. Biol. 9, 132 (2009).
Macbean, I.T. & Parsons, P.A. Directional selection for duration of copulation in Drosophila melanogaster. Genetics 56, 233–239 (1967).
Bretman, A., Fricke, C., Hetherington, P., Stone, R. & Chapman, T. Exposure to rivals and plastic responses to sperm competition in Drosophila melanogaster. Behav. Ecol. 21, 317–321 (2010).
von Lintig, J., Dreher, A., Kiefer, C., Wernet, M.F. & Vogt, K. Analysis of the blind Drosophila mutant ninaB identifies the gene encoding the key enzyme for vitamin A formation in vivo. Proc. Natl. Acad. Sci. USA 98, 1130–1135 (2001).
Cook, T., Pichaud, F., Sonneville, R., Papatsenko, D. & Desplan, C. Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila. Dev. Cell 4, 853–864 (2003).
Bretman, A., Westmancoat, J.D., Gage, M.J. & Chapman, T. Males use multiple, redundant cues to detect mating rivals. Curr. Biol. 21, 617–622 (2011).
Menne, D. & Spatz, H.C. Color vision in Drosophila melanogaster. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 114, 301–312 (1977).
Yamaguchi, S., Wolf, R., Desplan, C. & Heisenberg, M. Motion vision is independent of color in Drosophila. Proc. Natl. Acad. Sci. USA 105, 4910–4915 (2008).
Zheng, X. & Sehgal, A. Probing the relative importance of molecular oscillations in the circadian clock. Genetics 178, 1147–1155 (2008).
Hall, J.C. Systems approaches to biological rhythms in Drosophila. Methods Enzymol. 393, 61–185 (2005).
Beaver, L.M. & Giebultowicz, J.M. Regulation of copulation duration by period and timeless in Drosophila melanogaster. Curr. Biol. 14, 1492–1497 (2004).
Glaser, F.T. & Stanewsky, R. Temperature synchronization of the Drosophila circadian clock. Curr. Biol. 15, 1352–1363 (2005).
Emery, P., Stanewsky, R., Hall, J.C. & Rosbash, M. Drosophila cryptochromes: a unique circadian-rhythm photoreceptor. Nature 404, 456–457 (2000).
Yang, Z. & Sehgal, A. Role of molecular oscillations in generating behavioral rhythms in Drosophila. Neuron 29, 453–467 (2001).
Peschel, N. & Helfrich-Forster, C. Setting the clock—by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett. 585, 1435–1442.
Tanoue, S., Krishnan, P., Krishnan, B., Dryer, S.E. & Hardin, P.E. Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Curr. Biol. 14, 638–649 (2004).
Rieger, D., Wulbeck, C., Rouyer, F. & Helfrich-Forster, C. Period gene expression in four neurons is sufficient for rhythmic activity of Drosophila melanogaster under dim light conditions. J. Biol. Rhythms 24, 271–282 (2009).
Shafer, O.T., Helfrich-Forster, C., Renn, S.C. & Taghert, P.H. Reevaluation of Drosophila melanogaster's neuronal circadian pacemakers reveals new neuronal classes. J. Comp. Neurol. 498, 180–193 (2006).
Zhang, L. et al. DN1(p) circadian neurons coordinate acute light and PDF inputs to produce robust daily behavior in Drosophila. Curr. Biol. 20, 591–599 (2010).
Nitabach, M.N., Blau, J. & Holmes, T.C. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell 109, 485–495 (2002).
Nitabach, M.N. et al. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J. Neurosci. 26, 479–489 (2006).
Heisenberg, M. Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4, 266–275 (2003).
Popodi, E. et al. Small X duplications for the stock center collection. FlyBase published online, FBrf0210621 (2 March 2010).
Liu, G. et al. Distinct memory traces for two visual features in the Drosophila brain. Nature 439, 551–556 (2006).
Pan, Y. et al. Differential roles of the fan-shaped body and the ellipsoid body in Drosophila visual pattern memory. Learn. Mem. 16, 289–295 (2009).
Stocker, R.F. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 275, 3–26 (1994).
Renn, S.C. et al. Genetic analysis of the Drosophila ellipsoid body neuropil: organization and development of the central complex. J. Neurobiol. 41, 189–207 (1999).
Shang, Y., Griffith, L.C. & Rosbash, M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc. Natl. Acad. Sci. USA 105, 19587–19594 (2008).
Kent, C., Azanchi, R., Smith, B., Formosa, A. & Levine, J.D. Social context influences chemical communication in D. melanogaster males. Curr. Biol. 18, 1384–1389 (2008).
Krupp, J.J. et al. Social experience modifies pheromone expression and mating behavior in male. Drosophila melanogaster. Curr. Biol. 18, 1373–1383 (2008).
Gong, Z., Xia, S., Liu, L., Feng, C. & Guo, A. Operant visual learning and memory in Drosophila mutants dunce, amnesiac and radish. J. Insect Physiol. 44, 1149–1158 (1998).
Guo, A. & Gotz, K.G. Association of visual objects and olfactory cues in Drosophila. Learn. Mem. 4, 192–204 (1997).
Choi, C. & Nitabach, M.N. Circadian biology: environmental regulation of a multi-oscillator network. Curr. Biol. 20, R322–324 (2010).
Keene, A.C. et al. Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr. Biol. 20, 1209–1215 (2010).
Parisky, K.M. et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron 60, 672–682 (2008).
Krstic, D., Boll, W. & Noll, M. Sensory integration regulating male courtship behavior in Drosophila. PLoS ONE 4, e4457 (2009).
Preuss, F. et al. Drosophila doubletime mutations which either shorten or lengthen the period of circadian rhythms decrease the protein kinase activity of casein kinase I. Mol. Cell. Biol. 24, 886–898 (2004).
Hasegawa, E. et al. Concentric zones, cell migration and neuronal circuits in the Drosophila visual center. Development 138, 983–993 (2011).
Yang, C.H. et al. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 61, 519–526 (2009).
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999).
Acknowledgements
We thank S. Zhu for the unpublished fly line GAL414–94. We also thank A. Keene, J. Blau, M. Heisenberg, C. Helfrich-Förster, L. Griffith, R. Allada, M. Noll, J.L. Price, A. Sehgal, M. Young, J.D. Armstrong and M. Sato for kindly providing valuable flies. We are grateful to A. Keene for valuable discussion of this project and J. Berg for assisting with the writing of the manuscript. The work was supported by US National Institutes of Health grant 2R37NS040929 to Y.N.J. L.Y.J. and Y.N.J. are investigators of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
W.J.K. designed and performed the experiments. W.J.K., Y.N.J. and L.Y.J. wrote the manuscript. Y.N.J. and L.Y.J. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 682 kb)
Rights and permissions
About this article
Cite this article
Kim, W., Jan, L. & Jan, Y. Contribution of visual and circadian neural circuits to memory for prolonged mating induced by rivals. Nat Neurosci 15, 876–883 (2012). https://doi.org/10.1038/nn.3104
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3104
This article is cited by
-
A genetic screen for Drosophila social isolation mutants and analysis of sex pistol
Scientific Reports (2021)
-
Neuromodulatory circuit effects on Drosophila feeding behaviour and metabolism
Scientific Reports (2017)
-
Neuroethology of male courtship in Drosophila: from the gene to behavior
Journal of Comparative Physiology A (2014)
-
Genes and circuits of courtship behaviour in Drosophila males
Nature Reviews Neuroscience (2013)