Abstract
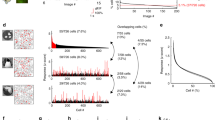
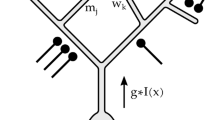
An influential theory of visual processing asserts that retinal center-surround receptive fields remove spatial correlations in the visual world, producing ganglion cell spike trains that are less redundant than the corresponding image pixels. For bright, high-contrast images, this decorrelation would enhance coding efficiency in optic nerve fibers of limited capacity. We tested the central prediction of the theory and found that the spike trains of retinal ganglion cells were indeed decorrelated compared with the visual input. However, most of the decorrelation was accomplished not by the receptive fields, but by nonlinear processing in the retina. We found that a steep response threshold enhanced efficient coding by noisy spike trains and that the effect of this nonlinearity was near optimal in both salamander and macaque retina. These results offer an explanation for the sparseness of retinal spike trains and highlight the importance of treating the full nonlinear character of neural codes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Attneave, F. Some informational aspects of visual perception. Psychol. Rev. 61, 183–193 (1954).
Barlow, H.B. Possible principles underlying the transformation of sensory messages. in Sensory Communication (ed. Rosenblith, W.A.) 217–234 (MIT Press, Cambridge, MA, 1961).
Srinivasan, M.V., Laughlin, S.B. & Dubs, A. Predictive coding: a fresh view of inhibition in the retina. Proc. R. Soc. Lond. B Biol. Sci. 216, 427–459 (1982).
Atick, J.J. & Redlich, A.N. What does the retina know about natural scenes? Neural Comput. 4, 196–210 (1992).
Atick, J.J. & Redlich, A.N. Convergent algorithm for sensory receptive field development. Neural Comput. 5, 45–60 (1993).
Atick, J.J. & Redlich, A.N. Could information theory provide an ecological theory of sensory processing? Network 3, 213–251 (1992).
van Hateren, J.H. Real and optimal neural images in early vision. Nature 360, 68–70 (1992).
van Hateren, J.H. Spatiotemporal contrast sensitivity of early vision. Vision Res. 33, 257–267 (1993).
Field, D.J. Relations between the statistics of natural images and the response properties of cortical cells. J. Opt. Soc. Am. A 4, 2379–2394 (1987).
Atick, J.J. & Redlich, A.N. Toward a theory of early visual processing. Neural Comput. 2, 308–320 (1990).
Dan, Y., Atick, J.J. & Reid, R.C. Efficient coding of natural scenes in the lateral geniculate nucleus: experimental test of a computational theory. J. Neurosci. 16, 3351–3362 (1996).
Puchalla, J.L., Schneidman, E., Harris, R.A. & Berry, M.J. Redundancy in the population code of the retina. Neuron 46, 493–504 (2005).
Chichilnisky, E.J. A simple white noise analysis of neuronal light responses. Network 12, 199–213 (2001).
Warland, D.K., Reinagel, P. & Meister, M. Decoding visual information from a population of retinal ganglion cells. J. Neurophysiol. 78, 2336–2350 (1997).
Segev, R., Puchalla, J. & Berry, M.J. Functional organization of ganglion cells in the salamander retina. J. Neurophysiol. 95, 2277–2292 (2006).
Enroth–Cugell, C. & Robson, J.G. Functional characteristics and diversity of cat retinal ganglion cells. Basic characteristics and quantitative description. Invest. Ophthalmol. Vis. Sci. 25, 250–267 (1984).
Berry, M.J. & Meister, M. Refractoriness and neural precision. J. Neurosci. 18, 2200–2211 (1998).
Burrone, J. & Lagnado, L. Synaptic depression and the kinetics of exocytosis in retinal bipolar cells. J. Neurosci. 20, 568–578 (2000).
Demb, J.B., Zaghloul, K., Haarsma, L. & Sterling, P. Bipolar cells contribute to nonlinear spatial summation in the brisk-transient (Y) ganglion cell in mammalian retina. J. Neurosci. 21, 7447–7454 (2001).
Field, G.D. & Rieke, F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron 34, 773–785 (2002).
Uzzell, V.J. & Chichilnisky, E.J. Precision of spike trains in primate retinal ganglion cells. J. Neurophysiol. 92, 780–789 (2004).
Pillow, J.W. et al. Spatio-temporal correlations and visual signaling in a complete neuronal population. Nature 454, 995–999 (2008).
Chichilnisky, E.J. & Kalmar, R.S. Functional asymmetries in ON and OFF ganglion cells of primate retina. J. Neurosci. 22, 2737–2747 (2002).
Croner, L.J., Purpura, K. & Kaplan, E. Response variability in retinal ganglion cells of primates. Proc. Natl. Acad. Sci. USA 90, 8128–8130 (1993).
Schwartz, O., Pillow, J.W., Rust, N.C. & Simoncelli, E.P. Spike-triggered neural characterization. J. Vis. 6, 484–507 (2006).
Lancaster, H.O. Some properties of the bivariate normal distribution considered in the form of a contingency table. Biometrika 44, 289–292 (1957).
de la Rocha, J., Doiron, B., Shea–Brown, E., Josic, K. & Reyes, A. Correlation between neural spike trains increases with firing rate. Nature 448, 802–806 (2007).
Berry, M.J., Warland, D.K. & Meister, M. The structure and precision of retinal spike trains. Proc. Natl. Acad. Sci. USA 94, 5411–5416 (1997).
Reinagel, P. How do visual neurons respond in the real world? Curr. Opin. Neurobiol. 11, 437–442 (2001).
Baccus, S.A. & Meister, M. Fast and slow contrast adaptation in retinal circuitry. Neuron 36, 909–919 (2002).
Stein, R.B. The information capacity of nerve cells using a frequency code. Biophys. J. 7, 797–826 (1967).
Shamai, S. Capacity of a pulse amplitude modulated direct detection photon channel. IEE Proc. Commun. Speech Vis. 137, 424–430 (1990).
Keat, J., Reinagel, P., Reid, R.C. & Meister, M. Predicting every spike: a model for the responses of visual neurons. Neuron 30, 803–817 (2001).
Balasubramanian, V. & Berry, M.J. A test of metabolically efficient coding in the retina. Network 13, 531–552 (2002).
Hosoya, T., Baccus, S.A. & Meister, M. Dynamic predictive coding by the retina. Nature 436, 71–77 (2005).
Croner, L.J. & Kaplan, E. Receptive fields of P and M ganglion cells across the primate retina. Vision Res. 35, 7–24 (1995).
Barlow, H.B. & Levick, W.R. The mechanism of directionally selective units in rabbit′s retina. J. Physiol. (Lond.) 178, 477–504 (1965).
Ölveczky, B.P., Baccus, S.A. & Meister, M. Segregation of object and background motion in the retina. Nature 423, 401–408 (2003).
Levick, W.R. Receptive fields and trigger features of ganglion cells in the visual streak of the rabbits retina. J. Physiol. (Lond.) 188, 285–307 (1967).
Gollisch, T. & Meister, M. Eye smarter than scientists believed: neural computations in circuits of the retina. Neuron 65, 150–164 (2010).
Dacey, D.M. Origins of perception: retinal ganglion cell diversity and the creation of parallel visual pathways. in The Cognitive Neurosciences (ed. Gazzaniga, M.S.) 281–301 (MIT Press, Cambridge, Massachusetts, 2004).
Laughlin, S.B. A simple coding procedure enhances a neuron′s information capacity. Z. Naturforsch. C 36c, 910–912 (1981).
Olshausen, B.A. & Field, D.J. Sparse coding of sensory inputs. Curr. Opin. Neurobiol. 14, 481–487 (2004).
Ringach, D.L. & Malone, B.J. The operating point of the cortex: neurons as large deviation detectors. J. Neurosci. 27, 7673–7683 (2007).
van Vreeswijk, C.A. Whence sparseness? Adv. Neural Inf. Process. Syst. 13, 189–195 (2001).
Vinje, W.E. & Gallant, J.L. Sparse coding and decorrelation in primary visual cortex during natural vision. Science 287, 1273–1276 (2000).
Wang, X.J., Liu, Y., Sanchez–Vives, M.V. & McCormick, D.A. Adaptation and temporal decorrelation by single neurons in the primary visual cortex. J. Neurophysiol. 89, 3279–3293 (2003).
Rucci, M. & Casile, A. Fixational instability and natural image statistics: implications for early visual representations. Network 16, 121–138 (2005).
Cleland, T.A. Early transformations in odor representation. Trends Neurosci. 33, 130–139 (2010).
Wiechert, M.T., Judkewitz, B., Riecke, H. & Friedrich, R.W. Mechanisms of pattern decorrelation by recurrent neuronal circuits. Nat. Neurosci. 13, 1003–1010 (2010).
Meister, M., Pine, J. & Baylor, D.A. Multi–neuronal signals from the retina: acquisition and analysis. J. Neurosci. Methods 51, 95–106 (1994).
Segev, R., Puchalla, J. & Berry, M.J. Functional organization of ganglion cells in the salamander retina. J. Neurophysiol. 95, 2277–2292 (2006).
Himstedt, W. Prey selection in salamanders. in Analysis of Visual Behavior (eds. Ingale, D.J., Goodale, M.A. & Mansfield, R.J.W.) 47–66 (MIT Press, Cambridge, Massachusetts, 1982).
Dong, D.W. & Atick, J.J. Statistics of natural time-varying images. Network 6, 345–358 (1995).
Croner, L.J. & Kaplan, E. Receptive fields of P and M ganglion cells across the primate retina. Vision Res. 35, 7–24 (1995).
Chichilnisky, E.J. & Kalmar, R.S. Functional asymmetries in ON and OFF ganglion cells of primate retina. J. Neurosci. 22, 2737–2747 (2002).
Berry, M.J. & Meister, M. Refractoriness and neural precision. J. Neurosci. 18, 2200–2211 (1998).
Uzzell, V.J. & Chichilnisky, E.J. Precision of spike trains in primate retinal ganglion cells. J. Neurophysiol. 92, 780–789 (2004).
Schneidman, E., Bialek, W. & Berry, M.J. Synergy, redundancy and independence in population codes. J. Neurosci. 23, 11539–11553 (2003).
Pillow, J.W. et al. Spatio–temporal correlations and visual signaling in a complete neuronal population. Nature 454, 995–999 (2008).
Acknowledgements
We thank the members of the Meister laboratory, M. Berry, T. Toyozumi and J.-P. Nadal for helpful advice. This work was funded by grants from the US National Institutes of Health to M.M.
Author information
Authors and Affiliations
Contributions
X.P. and M.M. designed the study. X.P. performed all of the experiments, analysis and modeling. X.P. and M.M. wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 501 kb)
Rights and permissions
About this article
Cite this article
Pitkow, X., Meister, M. Decorrelation and efficient coding by retinal ganglion cells. Nat Neurosci 15, 628–635 (2012). https://doi.org/10.1038/nn.3064
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3064
This article is cited by
-
Distinguishing examples while building concepts in hippocampal and artificial networks
Nature Communications (2024)
-
Active fixation as an efficient coding strategy for neuromorphic vision
Scientific Reports (2023)
-
Classical center-surround receptive fields facilitate novel object detection in retinal bipolar cells
Nature Communications (2022)
-
Efficient and adaptive sensory codes
Nature Neuroscience (2021)
-
Opposing effects of selectivity and invariance in peripheral vision
Nature Communications (2021)