Abstract
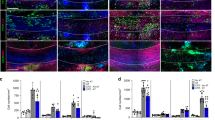
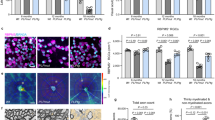
Anti-myelin immunity is commonly thought to drive multiple sclerosis, yet the initial trigger of this autoreactivity remains elusive. One of the proposed factors for initiating this disease is the primary death of oligodendrocytes. To specifically test such oligodendrocyte death as a trigger for anti-CNS immunity, we inducibly killed oligodendrocytes in an in vivo mouse model. Strong microglia-macrophage activation followed oligodendrocyte death, and myelin components in draining lymph nodes made CNS antigens available to lymphocytes. However, even conditions favoring autoimmunity—bystander activation, removal of regulatory T cells, presence of myelin-reactive T cells and application of demyelinating antibodies—did not result in the development of CNS inflammation after oligodendrocyte death. In addition, this lack of reactivity was not mediated by enhanced myelin-specific tolerance. Thus, in contrast with previously reported impairments of oligodendrocyte physiology, diffuse oligodendrocyte death alone or in conjunction with immune activation does not trigger anti-CNS immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Compston, A. & Coles, A. Multiple sclerosis. Lancet 372, 1502–1517 (2008).
Lassmann, H., Bruck, W. & Lucchinetti, C.F. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 17, 210–218 (2007).
Lucchinetti, C.F., Bruck, W. & Lassmann, H. Evidence for pathogenic heterogeneity in multiple sclerosis. Ann. Neurol. 56, 308 (2004).
Barnett, M.H. & Prineas, J.W. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann. Neurol. 55, 458–468 (2004).
Barnett, M.H. & Sutton, I. The pathology of multiple sclerosis: a paradigm shift. Curr. Opin. Neurol. 19, 242–247 (2006).
Filippi, M., Rocca, M.A., Martino, G., Horsfield, M.A. & Comi, G. Magnetization transfer changes in the normal appearing white matter precede the appearance of enhancing lesions in patients with multiple sclerosis. Ann. Neurol. 43, 809–814 (1998).
Narayana, P.A., Doyle, T.J., Lai, D. & Wolinsky, J.S. Serial proton magnetic resonance spectroscopic imaging, contrast-enhanced magnetic resonance imaging, and quantitative lesion volumetry in multiple sclerosis. Ann. Neurol. 43, 56–71 (1998).
Kassmann, C.M. et al. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 39, 969–976 (2007).
Ip, C.W. et al. Immune cells contribute to myelin degeneration and axonopathic changes in mice overexpressing proteolipid protein in oligodendrocytes. J. Neurosci. 26, 8206–8216 (2006).
Buch, T. et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat. Methods 2, 419–426 (2005).
Hövelmeyer, N. et al. Apoptosis of oligodendrocytes via Fas and TNF-R1 is a key event in the induction of experimental autoimmune encephalomyelitis. J. Immunol. 175, 5875–5884 (2005).
Traka, M. et al. A genetic mouse model of adult-onset, pervasive central nervous system demyelination with robust remyelination. Brain 133, 3017–3029 (2010).
Pohl, H.B. et al. Genetically induced adult oligodendrocyte cell death is associated with poor myelin clearance, reduced remyelination, and axonal damage. J. Neurosci. 31, 1069–1080 (2011).
van Zwam, M. et al. Surgical excision of CNS-draining lymph nodes reduces relapse severity in chronic-relapsing experimental autoimmune encephalomyelitis. J. Pathol. 217, 543–551 (2009).
de Vos, A.F. et al. Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. J. Immunol. 169, 5415–5423 (2002).
Fabriek, B.O. et al. In vivo detection of myelin proteins in cervical lymph nodes of MS patients using ultrasound-guided fine-needle aspiration cytology. J. Neuroimmunol. 161, 190–194 (2005).
Bettelli, E. et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197, 1073–1081 (2003).
Frommer, F. et al. Tolerance without clonal expansion: self-antigen-expressing B cells program self-reactive T cells for future deletion. J. Immunol. 181, 5748–5759 (2008).
Elgueta, R. et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229, 152–172 (2009).
Ransohoff, R.M. & Perry, V.H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145 (2009).
Carson, M.J. Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia 40, 218–231 (2002).
Becher, B. & Antel, J.P. Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia 18, 1–10 (1996).
Link, H. & Muller, R. Immunoglobulins in multiple sclerosis and infections of the nervous system. Arch. Neurol. 25, 326–344 (1971).
Herndon, R.M. & Kasckow, J. Electron microscopic studies of cerebrospinal fluid sediment in demyelinating disease. Ann. Neurol. 4, 515–523 (1978).
Prineas, J.W. & Wright, R.G. Macrophages, lymphocytes, and plasma cells in the perivascular compartment in chronic multiple sclerosis. Lab. Invest. 38, 409–421 (1978).
Urich, E., Gutcher, I., Prinz, M. & Becher, B. Autoantibody-mediated demyelination depends on complement activation, but not activatory Fc-receptors. Proc. Natl. Acad. Sci. USA 103, 18697–18702 (2006).
Gallucci, S. & Matzinger, P. Danger signals: SOS to the immune system. Curr. Opin. Immunol. 13, 114–119 (2001).
Mueller, D.L. Mechanisms maintaining peripheral tolerance. Nat. Immunol. 11, 21–27 (2010).
Sakaguchi, S. et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212, 8–27 (2006).
Honjo, T., Nishizuka, Y., Kato, I. & Hayaishi, O. Adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis by diphtheria toxin. J. Biol. Chem. 246, 4251–4260 (1971).
Emerson, M.R., Biswas, S. & Le Vine, S.M. Cuprizone and piperonyl butoxide, proposed inhibitors of T-cell function, attenuate experimental allergic encephalomyelitis in SJL mice. J. Neuroimmunol. 119, 205–213 (2001).
Zatta, P. et al. Copper and zinc dismetabolism in the mouse brain upon chronic cuprizone treatment. Cell. Mol. Life Sci. 62, 1502–1513 (2005).
Elloso, M.M., Phiel, K., Henderson, R.A., Harris, H.A. & Adelman, S.J. Suppression of experimental autoimmune encephalomyelitis using estrogen receptor-selective ligands. J. Endocrinol. 185, 243–252 (2005).
Chernoff, G.F. Shiverer: an autosomal recessive mutant mouse with myelin deficiency. J. Hered. 72, 128 (1981).
Wang, H., Zhan, Y., Xu, L., Feuerstein, G.Z. & Wang, X. Use of suppression subtractive hybridization for differential gene expression in stroke: discovery of CD44 gene expression and localization in permanent focal stroke in rats. Stroke 32, 1020–1027 (2001).
Kim, M.D., Cho, H.J. & Shin, T. Expression of osteopontin and its ligand, CD44, in the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis. J. Neuroimmunol. 151, 78–84 (2004).
Bédard, A., Tremblay, P., Chernomoretz, A. & Vallieres, L. Identification of genes preferentially expressed by microglia and upregulated during cuprizone-induced inflammation. Glia 55, 777–789 (2007).
Remington, L.T., Babcock, A.A., Zehntner, S.P. & Owens, T. Microglial recruitment, activation and proliferation in response to primary demyelination. Am. J. Pathol. 170, 1713–1724 (2007).
Hiremath, M.M., Chen, V.S., Suzuki, K., Ting, J.P. & Matsushima, G.K. MHC class II exacerbates demyelination in vivo independently of T cells. J. Neuroimmunol. 203, 23–32 (2008).
Adams, C.W., Poston, R.N. & Buk, S.J. Pathology, histochemistry and immunocytochemistry of lesions in acute multiple sclerosis. J. Neurol. Sci. 92, 291–306 (1989).
Maña, P. et al. Demyelination caused by the copper chelator cuprizone halts T cell–mediated autoimmune neuroinflammation. J. Neuroimmunol. 210, 13–21 (2009).
Griffiths, I. et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 280, 1610–1613 (1998).
Yin, X. et al. Evolution of a neuroprotective function of central nervous system myelin. J. Cell Biol. 172, 469–478 (2006).
Lappe-Siefke, C. et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 33, 366–374 (2003).
Berger, J. & Gartner, J. X-linked adrenoleukodystrophy: clinical, biochemical and pathogenetic aspects. Biochim. Biophys. Acta 1763, 1721–1732 (2006).
Haak, S. et al. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J. Clin. Invest. 119, 61–69 (2009).
Weston, S.A. & Parish, C.R. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J. Immunol. Methods 133, 87–97 (1990).
Becher, B., Durell, B.G. & Noelle, R.J. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Invest. 110, 493–497 (2002).
Förster, I. & Rajewsky, K. Expansion and functional activity of Ly-1 + B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur. J. Immunol. 17, 521–528 (1987).
Spergel, D.J., Kruth, U., Hanley, D.F., Sprengel, R. & Seeburg, P.H. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J. Neurosci. 19, 2037–2050 (1999).
Acknowledgements
We are grateful for technical support to V. Wörtmann, A. Apladas, M. Perkovic, the Zentrum für Mikroskopie und Bildanalyse, T. Bruggmann, J. Huppert and K. Fischer, as well as the mouse facility teams in Zürich and Mainz. We thank J.D. Laman and I. Parvanova for help with manuscript preparation. This work was supported by COST action BM603 NEURINFNET (B.B., T.B., G.L. and I.B.), by Deutsche Forschungsgemeinschaft (DFG) grants 1600/3-1 and SFB/TR 52, and the German Ministry for Education and Research (Consortium UNDERSTANDMS/German Competence Network of Multiple Sclerosis) to A.W., by the DFG grant FOR1336 to I.B. and A.W., by a grant from the Bonizzi-Theler foundation to T.B., by the State Secretariat for Education and Research of Switzerland to T.B. and B.B., by the Schweizerische Nationalfonds (SNF) to T.B. (CRSI33_125073) and B.B., the National Center of Competence in Research (NCCR-Neuro) to B.B., the Swiss Multiple Sclerosis Society to T.B., and by DFG grants SFB TR43 and Exc 25 and the US National Institutes of Health (National Institute of Neurological Disorders and Stroke R01 NS046006) to F.L.H.
Author information
Authors and Affiliations
Contributions
T.B., B.B. and A.W. conceived the experimental system. G.L., T.B., S.W., B.I., M.K., K.K., C.B., I.B., B.S. and F.F. carried out the experiments and analyzed the data. T.B., F.L.H., A.W. and B.B. supervised the work. G.L. and T.B. co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 476 kb)
Supplementary Video 1
Clinical manifestation of ataxia and tremor during motor tests following ODC death. oDTR mice were treated with DTx. The movie shows the starting phase of the clinical disease 5 weeks p.a.. Mice showed progressive ataxia which manifested itself as inability to place paws correctly during the grid walking. Also, strong tremor could be observed (score 2 in our 0–3 scale). (MOV 1116 kb)
Supplementary Video 2
Progression of clinical disease leads to strong kyphosis and dystonia. oDTR mice were treated with DTx. The movie shows the progression of the clinical disease at around 6 weeks p.a.. Mice showed kyphosis, dystonia of hind limbs, inability to maintain balanced position. (MOV 211 kb)
Rights and permissions
About this article
Cite this article
Locatelli, G., Wörtge, S., Buch, T. et al. Primary oligodendrocyte death does not elicit anti-CNS immunity. Nat Neurosci 15, 543–550 (2012). https://doi.org/10.1038/nn.3062
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3062
This article is cited by
-
Gateway reflexes describe novel neuro-immune communications that establish immune cell gateways at specific vessels
Bioelectronic Medicine (2023)
-
Novel Immunotherapeutic Approaches for the Treatment of Glioblastoma
BioDrugs (2023)
-
Microglia and monocytes in inflammatory CNS disease: integrating phenotype and function
Acta Neuropathologica (2022)
-
Myelin replacement triggered by single-cell demyelination in mouse cortex
Nature Communications (2020)
-
Missing in Action: Dysfunctional RNA Metabolism in Oligodendroglial Cells as a Contributor to Neurodegenerative Diseases?
Neurochemical Research (2019)