Abstract
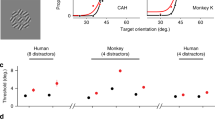
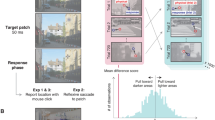
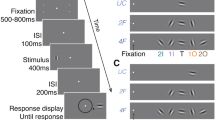
Processing of shape information in human peripheral visual fields is impeded beyond what can be expected by poor spatial resolution. Visual crowding, the inability to identify objects in clutter, has been shown to be the primary factor limiting shape perception in peripheral vision. Despite the well-documented effects of crowding, its underlying causes remain poorly understood. Given that spatial attention both facilitates learning of image statistics and directs saccadic eye movements, we propose that the acquisition of image statistics in peripheral visual fields is confounded by eye-movement artifacts. Specifically, the image statistics acquired under a peripherally deployed spotlight of attention are systematically biased by saccade-induced image displacements. These erroneously represented image statistics lead to inappropriate contextual interactions in the periphery and cause crowding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Levi, D.M. Crowding—an essential bottleneck for object recognition: a mini-review. Vision Res. 48, 635–654 (2008).
Bouma, H. Interaction effects in parafoveal letter recognition. Nature 226, 177–178 (1970).
Levi, D.M., Hariharan, S. & Klein, S. Suppressive and facilitatory spatial interactions in peripheral vision: peripheral crowding is neither size invariant nor simple contrast masking. J. Vis. 2, 167–177 (2002).
Pelli, D.G., Palomares, M.C. & Majaj, N.J. Crowding is unlike ordinary masking: distinguishing feature integration from detection. J. Vis. 4, 1136–1169 (2004).
Nandy, A.S. & Tjan, B.S. The nature of letter crowding as revealed by first- and second-order classification images. J. Vis. 7, 1–26 (2007).
Greenwood, J.A., Bex, P. & Dakin, S.C. Positional averaging explains crowding with letter-like stimuli. Proc. Natl. Acad. Sci. USA 106, 13130–13135 (2009).
He, S., Cavanagh, P. & Intriligator, J. Attentional resolution and the locus of visual awareness. Nature 383, 334–337 (1996).
Petrov, Y., Popple, A.V. & McKee, S.P. Crowding and surround suppression: not to be confused. J. Vis. 7, 1–9 (2007).
Toet, A. & Levi, D.M. The two-dimensional shape of spatial interaction zones in the parafovea. Vision Res. 32, 1349–1357 (1992).
Pelli, D.G. Crowding: a cortical constraint on object recognition. Curr. Opin. Neurobiol. 18, 445–451 (2008).
Motter, B.C. & Simoni, D.A. The roles of cortical image separation and size in active visual search performance. J. Vis. 7, 1–15 (2007).
Petrov, Y. & Popple, A.V. Crowding is directed to the fovea and preserves only feature contrast. J. Vis. 7, 1–9 (2007).
van den Berg, R. & Roerdink, J.B.T.M. & Cornelissen, F.W. A neurophysiologically plausible population code model for feature integration explains visual crowding. PLoS Comput. Biol. 6, e1000646 (2010).
Geisler, W.S. Visual perception and the statistical properties of natural scenes. Annu. Rev. Psychol. 59, 167–192 (2008).
Karklin, Y. & Lewicki, M.S. Emergence of complex cell properties by learning to generalize in natural scenes. Nature 457, 83–86 (2009).
Olshausen, B.A. & Field, D.J. Emergence of simple-cell receptive field properties by learning a sparse code for natural images. Nature 381, 607–609 (1996).
Kapadia, M.K., Ito, M., Gilbert, C.D. & Westheimer, G. Improvement in visual sensitivity by changes in local context: parallel studies in human observers and in V1 of alert monkeys. Neuron 15, 843–856 (1995).
Stettler, D.D., Das, A., Bennett, J. & Gilbert, C.D. Lateral connectivity and contextual interactions in macaque primary visual cortex. Neuron 36, 739–750 (2002).
Sigman, M., Cecchi, G.A., Gilbert, C.D. & Magnasco, M.O. On a common circle: natural scenes and Gestalt rules. Proc. Natl. Acad. Sci. USA 98, 1935–1940 (2001).
Geisler, W.S., Perry, J.S., Super, B.J. & Gallogly, D.P. Edge co-occurrence in natural images predicts contour grouping performance. Vision Res. 41, 711–724 (2001).
Ito, M. & Gilbert, C.D. Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron 22, 593–604 (1999).
Gilbert, C., Ito, M., Kapadia, M.K. & Westheimer, G. Interactions between attention, context and learning in primary visual cortex. Vision Res. 40, 1217–1226 (2000).
Deubel, H. & Schneider, W.X. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 36, 1827–1837 (1996).
Motter, B.C. Crowding and object integration within the receptive field of V4 neurons. J. Vis. 2, 274 (2002).
Ben-Shahar, O. & Zucker, S. Geometrical computations explain projection patterns of long-range horizontal connections in visual cortex. Neural Comput. 16, 445–476 (2004).
Bosking, W.H., Zhang, Y., Schofield, B. & Fitzpatrick, D. Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. J. Neurosci. 17, 2112–2127 (1997).
Greenwood, J.A., Bex, P.J. & Dakin, S.C. Crowding changes appearance. Curr. Biol. 20, 496–501 (2010).
Parkes, L., Lund, J., Angelucci, A., Solomon, J.A. & Morgan, M. Compulsory averaging of crowded orientation signals in human vision. Nat. Neurosci. 4, 739–744 (2001).
Levi, D.M. & Carney, T. Crowding in peripheral vision: why bigger is better. Curr. Biol. 19, 1988–1993 (2009).
Freeman, J. & Simoncelli, E.P. Metamers of the ventral stream. Nat. Neurosci. 14, 1195–1201 (2011).
Bahill, A.T., Adler, D. & Stark, L. Most naturally occurring human saccades have magnitudes of 15 degrees or less. Invest. Ophthalmol. Vis. Sci. 14, 68–69 (1975).
Levi, D.M., Song, S. & Pelli, D.G. Amblyopic reading is crowded. J. Vis. 7, 1–17 (2007).
Bruce, N.D.B. & Tsotsos, J.K. A statistical basis for visual field anisotropies. Neurocomputing 69, 1301–1304 (2006).
White, J.M. & Bedell, H.E. The oculomotor reference in humans with bilateral macular disease. Invest. Ophthalmol. Vis. Sci. 31, 1149–1161 (1990).
Neri, P. & Levi, D.M. Spatial resolution for feature binding is impaired in peripheral and amblyopic vision. J. Neurophysiol. 96, 142–153 (2006).
Itti, L. & Koch, C. Computational modeling of visual attention. Nat. Rev. Neurosci. 2, 194–203 (2001).
Diamond, M.R., Ross, J. & Morrone, M.C. Extraretinal control of saccadic suppression. J. Neurosci. 20, 3449–3455 (2000).
Campbell, F.W. & Wurtz, R.H. Saccadic omission: why we do not see a grey-out during a saccadic eye movement. Vision Res. 18, 1297–1303 (1978).
Wurtz, R.H. Neuronal mechanisms of visual stability. Vision Res. 48, 2070–2089 (2008).
García-Pérez, M.A. & Peli, E. Intrasaccadic perception. J. Neurosci. 21, 7313–7322 (2001).
De Pisapia, N., Kaunitz, L. & Melcher, D. Backward masking and unmasking across saccadic eye movements. Curr. Biol. 20, 613–617 (2010).
Watson, T.L. & Krekelberg, B. The relationship between saccadic suppression and perceptual stability. Curr. Biol. 19, 1040–1043 (2009).
Castet, E., Jeanjean, S. & Masson, G.S. Motion perception of saccade-induced retinal translation. Proc. Natl. Acad. Sci. USA 99, 15159–15163 (2002).
Gattass, R., Gross, C.G. & Sandell, J.H. Visual topography of V2 in the macaque. J. Comp. Neurol. 201, 519–539 (1981).
Piñon, M.C., Gattass, R. & Sousa, A.P. Area V4 in Cebus monkey: extent and visuotopic organization. Cereb. Cortex 8, 685–701 (1998).
Motter, B.C. Central V4 receptive fields are scaled by the V1 cortical magnification and correspond to a constant-sized sampling of the V1 surface. J. Neurosci. 29, 5749–5757 (2009).
Whitney, D. & Levi, D.M. Visual crowding: a fundamental limit on conscious perception and object recognition. Trends Cogn. Sci. 15, 160–168 (2011).
Larsson, J. & Heeger, D.J. Two retinotopic visual areas in human lateral occipital cortex. J. Neurosci. 26, 13128–13142 (2006).
Wilson, J.R. & Sherman, S.M. Receptive-field characteristics of neurons in cat striate cortex: changes with visual field eccentricity. J. Neurophysiol. 39, 512–533 (1976).
Lebedev, S., Gelder, P.V. & Tsui, W.H. Square-root relations between main saccadic parameters. Invest. Ophthalmol. Vis. Sci. 37, 2750–2758 (1996).
Acknowledgements
The authors would like to thank I. Biederman, A. Disney, J. Hirsch, M. Jadi, R. Millin and J. Reynolds for helpful comments on the manuscript. This research was supported by US National Institutes of Health grant EY017707 (B.S.T.).
Author information
Authors and Affiliations
Contributions
A.S.N. and B.S.T. developed the theory, designed the experiments and collected the data. A.S.N. analyzed the data and ran the model simulations. A.S.N. and B.S.T. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Table 1 and Supplementary Note (PDF 6792 kb)
Rights and permissions
About this article
Cite this article
Nandy, A., Tjan, B. Saccade-confounded image statistics explain visual crowding. Nat Neurosci 15, 463–469 (2012). https://doi.org/10.1038/nn.3021
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3021
This article is cited by
-
The Role of Attention in the Recognition of Peripheral Stimuli in Single and Dual Tasks
Neuroscience and Behavioral Physiology (2022)
-
Mixture model investigation of the inner–outer asymmetry in visual crowding reveals a heavier weight towards the visual periphery
Scientific Reports (2021)
-
Spatial contextual effects in primary visual cortex limit feature representation under crowding
Nature Communications (2020)
-
Perceptual integration and attention in human extrastriate cortex
Scientific Reports (2017)
-
Age-related changes in crowding and reading speed
Scientific Reports (2017)