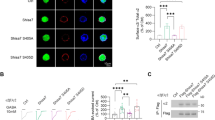
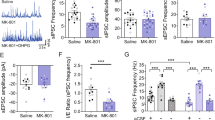
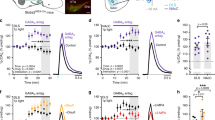
Abstract
The striatum regulates motor control, reward and learning. Abnormal function of striatal GABAergic medium spiny neurons (MSNs) is believed to contribute to the deficits in these processes that are observed in many neuropsychiatric diseases. The orphan G protein–coupled receptor GPR88 is robustly expressed in MSNs and is regulated by neuropharmacological drugs, but its contribution to MSN physiology and behavior is unclear. We found that, in the absence of GPR88, MSNs showed increased glutamatergic excitation and reduced GABAergic inhibition, which promoted enhanced firing rates in vivo, resulting in hyperactivity, poor motor coordination and impaired cue-based learning in mice. Targeted viral expression of GPR88 in MSNs rescued the molecular and electrophysiological properties and normalized behavior, suggesting that aberrant MSN activation in the absence of GPR88 underlies behavioral deficits and its dysfunction may contribute to behaviors observed in neuropsychiatric disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Gerfen, C.R. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu. Rev. Neurosci. 15, 285–320 (1992).
Surmeier, D.J., Plotkin, J. & Shen, W. Dopamine and synaptic plasticity in dorsal striatal circuits controlling action selection. Curr. Opin. Neurobiol. 19, 621–628 (2009).
Balleine, B.W., Delgado, M.R. & Hikosaka, O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 27, 8161–8165 (2007).
Drew, M.R. et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J. Neurosci. 27, 7731–7739 (2007).
Russell, V.A. Neurobiology of animal models of attention-deficit hyperactivity disorder. J. Neurosci. Methods 161, 185–198 (2007).
Graybiel, A.M., Aosaki, T., Flaherty, A.W. & Kimura, M. The basal ganglia and adaptive motor control. Science 265, 1826–1831 (1994).
Joshi, P.R. et al. Age-dependent alterations of corticostriatal activity in the YAC128 mouse model of Huntington disease. J. Neurosci. 29, 2414–2427 (2009).
Massart, R., Guilloux, J.P., Mignon, V., Sokoloff, P. & Diaz, J. Striatal GPR88 expression is confined to the whole projection neuron population and is regulated by dopaminergic and glutamatergic afferents. Eur. J. Neurosci. 30, 397–414 (2009).
Böhm, C. et al. Effects of antidepressant treatment on gene expression profile in mouse brain: cell type–specific transcription profiling using laser microdissection and microarray analysis. J. Neurochem. 97 (suppl. 1), 44–49 (2006).
Conti, B. et al. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Mol. Psychiatry 12, 167–189 (2007).
Befort, K. et al. Mu-opioid receptor activation induces transcriptional plasticity in the central extended amygdala. Eur. J. Neurosci. 27, 2973–2984 (2008).
Logue, S.F. et al. The orphan GPCR, GPR88, modulates function of the striatal dopamine system: a possible therapeutic target for psychiatric disorders? Mol. Cell Neurosci. 42, 438–447 (2009).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Sanz, E. et al. Cell type–specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl. Acad. Sci. USA 106, 13939–13944 (2009).
Darvas, M. & Palmiter, R.D. Restricting dopaminergic signaling to either dorsolateral or medial striatum facilitates cognition. J. Neurosci. 30, 1158–1165 (2010).
McDonald, R.J. & White, N.M. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav. Neural Biol. 61, 260–270 (1994).
Packard, M.G. & Knowlton, B.J. Learning and memory functions of the basal ganglia. Annu. Rev. Neurosci. 25, 563–593 (2002).
Darvas, M. & Palmiter, R.D. Contributions of striatal dopamine signaling to the modulation of cognitive flexibility. Biol. Psychiatry 69, 704–707 (2011).
van der Heyden, J.A. & Bradford, L.D. A rapidly acquired one-way conditioned avoidance procedure in rats as a primary screening test for antipsychotics: influence of shock intensity on avoidance performance. Behav. Brain Res. 31, 61–67 (1988).
Berke, J.D., Okatan, M., Skurski, J. & Eichenbaum, H.B. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43, 883–896 (2004).
Wilson, C.J. & Kawaguchi, Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J. Neurosci. 16, 2397–2410 (1996).
Núñez-Abades, P.A., Pattillo, J.M., Hodgson, T.M. & Cameron, W.E. Role of synaptic inputs in determining input resistance of developing brain stem motoneurons. J. Neurophysiol. 84, 2317–2329 (2000).
Kravitz, A.V. et al. Regulation of parkinsonian motor behaviors by optogenetic control of basal ganglia circuitry. Nature 466, 622–626 (2010).
Ferguson, S.M. & Neumaier, J.F. Grateful DREADDs: engineered receptors reveal how neural circuits regulate behavior. Neuropsychopharmacology 37, 296–297 (2012).
Lobo, M.K. et al. Cell type–specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330, 385–390 (2010).
Wang, W. et al. Regulation of prefrontal excitatory neurotransmission by dopamine in the nucleus accumbens core. J. Physiol. (Lond.) 590, 3743–3769 (2012).
Zhang, F. et al. Association analyses of the interaction between the ADSS and ATM genes with schizophrenia in a Chinese population. BMC Med. Genet. 9, 119 (2008).
Chen, X. et al. FBXL21 association with schizophrenia in Irish family and case-control samples. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 147B, 1231–1237 (2008).
Chowdari, K.V. et al. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum. Mol. Genet. 11, 1373–1380 (2002).
Berman, D.M., Wilkie, T.M. & Gilman, A.G. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell 86, 445–452 (1996).
Ding, J. et al. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat. Neurosci. 9, 832–842 (2006).
Corvin, A.P. et al. Confirmation and refinement of an 'at-risk' haplotype for schizophrenia suggests the EST cluster, Hs.97362, as a potential susceptibility gene at the Neuregulin-1 locus. Mol. Psychiatry 9, 208–213 (2004).
Lerner, T.N. & Kreitzer, A.C. RGS4 is required for dopaminergic control of striatal LTD and susceptibility to Parkinsonian motor deficits. Neuron 73, 347–359 (2012).
Song, I. & Huganir, R.L. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 25, 578–588 (2002).
Jacob, T.C., Moss, S.J. & Jurd, R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 9, 331–343 (2008).
Janssen, M.J., Ade, K.K., Fu, Z. & Vicini, S. Dopamine modulation of GABA tonic conductance in striatal output neurons. J. Neurosci. 29, 5116–5126 (2009).
Parker, J.G. et al. Attenuating GABA(A) receptor signaling in dopamine neurons selectively enhances reward learning and alters risk preference in mice. J. Neurosci. 31, 17103–17112 (2011).
Ade, K.K., Janssen, M.J., Ortinski, P.I. & Vicini, S. Differential tonic GABA conductances in striatal medium spiny neurons. J. Neurosci. 28, 1185–1197 (2008).
Farrant, M. & Nusser, Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 6, 215–229 (2005).
Zweifel, L.S. et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc. Natl. Acad. Sci. USA 106, 7281–7288 (2009).
Nicola, S.M., Surmeier, J. & Malenka, R.C. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu. Rev. Neurosci. 23, 185–215 (2000).
Surmeier, D.J. et al. The role of dopamine in modulating the structure and function of striatal circuits. Prog. Brain Res. 183, 149–167 (2010).
Ralph-Williams, R.J., Lehmann-Masten, V., Otero-Corchon, V., Low, M.J. & Geyer, M.A. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knockout mice. J. Neurosci. 22, 9604–9611 (2002).
Kodsi, M.H. & Swerdlow, N.R. Quinolinic acid lesions of the ventral striatum reduce sensorimotor gating of acoustic startle in rats. Brain Res. 643, 59–65 (1994).
Braff, D.L., Geyer, M.A. & Swerdlow, N.R. Human studies of prepulse inhibition of startle: normal subjects, patient groups and pharmacological studies. Psychopharmacology (Berl.) 156, 234–258 (2001).
Brandish, P.E. et al. Regulation of gene expression by lithium and depletion of inositol in slices of adult rat cortex. Neuron 45, 861–872 (2005).
Ogden, C.A. et al. Candidate genes, pathways and mechanisms for bipolar (manic-depressive) and related disorders: an expanded convergent functional genomics approach. Mol. Psychiatry 9, 1007–1029 (2004).
Farley, F.W., Soriano, P., Steffen, L.S. & Dymecki, S.M. Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28, 106–110 (2000).
Quintana, A., Kruse, S.E., Kapur, R.P., Sanz, E. & Palmiter, R.D. Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc. Natl. Acad. Sci. USA 107, 10996–11001 (2010).
Perez, F.A. & Palmiter, R.D. Parkin-deficient mice are not a robust model of parkinsonism. Proc. Natl. Acad. Sci. USA 102, 2174–2179 (2005).
Darvas, M., Fadok, J.P. & Palmiter, R.D. Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learn. Mem. 18, 136–143 (2011).
Duncan, D.T., Prodduturi, N. & Zhang, B. WebGestalt2: an updated and expanded version of the Web-based Gene Set Analysis Toolkit. BMC Bioinformatics 11, P10 (2010).
Ariano, M.A. et al. Striatal potassium channel dysfunction in Huntington′s disease transgenic mice. J. Neurophysiol. 93, 2565–2574 (2005).
Mermelstein, P.G., Song, W.J., Tkatch, T., Yan, Z. & Surmeier, D.J. Inwardly rectifying potassium (IRK) currents are correlated with IRK subunit expression in rat nucleus accumbens medium spiny neurons. J. Neurosci. 18, 6650–6661 (1998).
Quintana, A. et al. Fatal breathing dysfunction in a mouse model of Leigh syndrome. J. Clin. Invest. 122, 2359–2368 (2012).
Acknowledgements
The authors thank G. Froelick for assistance with histology, J. Parker for help making the targeting construct, L. Zweifel and M. Carter for helpful discussions, and D. Durnam for editing. This work was supported by grants NS052536, NS060803, HD02274 (N.S.B.), MH086386 (G.S.M. and P.S.A.), DA007278 (G.P.S.) and GM032875 (G.S.M.) from the US National Institutes of Health. A.Q. and E.S. were recipients of Spanish Ministry of Science and Innovation postdoctoral mobility program fellowships.
Author information
Authors and Affiliations
Contributions
A.Q. and R.D.P. designed the study. R.D.P. generated the Gpr88Cre/Cre mice. A.Q. performed the histological experiments. A.Q. and E.S. performed the RiboTag and biochemical experiments. E.S. performed the microarray experiment and A.Q. analyzed the data. W.W., G.P.S. and M.J.W. performed the in vitro electrophysiological studies and analyzed the data. A.Q. and A.D.G. performed the in vivo electrophysiological experiments and A.D.G. analyzed the data. A.Q. and B.A.R. performed the behavioral experiments and analyzed the data. A.Q. made the viral vectors and performed stereotaxic surgery. A.L.T. performed the neuronal primary culture experiments. P.S.A. and G.S.M. supervised the biochemical experiments. N.S.B. supervised the electrophysiological experiments. R.D.P. supervised the study. A.Q., N.S.B. and R.D.P. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 1–4 (PDF 3892 kb)
Rights and permissions
About this article
Cite this article
Quintana, A., Sanz, E., Wang, W. et al. Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue-dependent behaviors. Nat Neurosci 15, 1547–1555 (2012). https://doi.org/10.1038/nn.3239
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3239
This article is cited by
-
Neonatal immune challenge poses a sex-specific risk for epigenetic microglial reprogramming and behavioral impairment
Nature Communications (2023)
-
Update on GPCR-based targets for the development of novel antidepressants
Molecular Psychiatry (2022)
-
The orphan receptor GPR88 controls impulsivity and is a risk factor for Attention-Deficit/Hyperactivity Disorder
Molecular Psychiatry (2022)
-
Transcriptional signatures in prefrontal cortex confer vulnerability versus resilience to food and cocaine addiction-like behavior
Scientific Reports (2021)
-
Deletion of the clock gene Period2 (Per2) in glial cells alters mood-related behavior in mice
Scientific Reports (2021)