Abstract
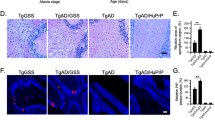
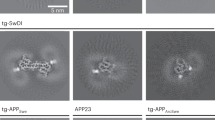
The amyloid-β peptide Aβ42 is known to be a primary amyloidogenic and pathogenic agent in Alzheimer's disease. However, the role of Aβ43, which is found just as frequently in the brains of affected individuals, remains unresolved. We generated knock-in mice containing a pathogenic presenilin-1 R278I mutation that causes overproduction of Aβ43. Homozygosity was embryonic lethal, indicating that the mutation involves a loss of function. Crossing amyloid precursor protein transgenic mice with heterozygous mutant mice resulted in elevated Aβ43, impairment of short-term memory and acceleration of amyloid-β pathology, which accompanied pronounced accumulation of Aβ43 in plaque cores similar in biochemical composition to those observed in the brains of affected individuals. Consistently, Aβ43 showed a higher propensity to aggregate and was more neurotoxic than Aβ42. Other pathogenic presenilin mutations also caused overproduction of Aβ43 in a manner correlating with Aβ42 and with the age of disease onset. These findings indicate that Aβ43, an overlooked species, is potently amyloidogenic, neurotoxic and abundant in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Blennow, K., de Leon, M.J. & Zetterberg, H. Alzheimer's disease. Lancet 368, 387–403 (2006).
Miravalle, L. et al. Amino-terminally truncated Aβ peptide species are the main component of cotton wool plaques. Biochemistry 44, 10810–10821 (2005).
Van Vickle, G.D. et al. TgCRND8 amyloid precursor protein transgenic mice exhibit an altered γ-secretase processing and an aggressive, additive amyloid pathology subject to immunotherapeutic modulation. Biochemistry 46, 10317–10327 (2007).
Iizuka, T. et al. Amyloid β-protein ending at Thr43 is a minor component of some diffuse plaques in the Alzheimer's disease brain, but is not found in cerebrovascular amyloid. Brain Res. 702, 275–278 (1995).
Parvathy, S. et al. Correlation between Aßx-40-, Aßx-42- and Aßx-43-containing amyloid plaques and cognitive decline. Arch. Neurol. 58, 2025–2032 (2001).
Welander, H. et al. Aβ43 is more frequent than Aβ40 in amyloid plaque cores from Alzheimer disease brains. J. Neurochem. 110, 697–706 (2009).
Keller, L. et al. The PSEN1 I143T mutation in a Swedish family with Alzheimer's disease: clinical report and quantification of Aβ in different brain regions. Eur. J. Hum. Genet. 18, 1202–1208 (2010).
Qi-Takahara, Y. et al. Longer forms of amyloid β protein: implications for the mechanism of intramembrane cleavage by γ-secretase. J. Neurosci. 25, 436–445 (2005).
Takami, M. et al. γ-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of β-carboxyl terminal fragment. J. Neurosci. 29, 13042–13052 (2009).
Shimojo, M. et al. Enzymatic characteristics of I213T mutant Presenilin-1/γ-secretase in cell models and knock-in mouse brains: FAD-linked mutation impairs γ-site cleavage of APP-CTFβ. J. Biol. Chem. 283, 16488–16496 (2008).
Jarrett, J.T., Berger, E.P. & Lansbury, P.T. Jr. The carboxy terminus of the β amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry 32, 4693–4697 (1993).
Bitan, G. et al. Amyloid β-protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci. USA 100, 330–335 (2003).
Nakaya, Y. et al. Random mutagenesis of presenilin-1 identifies novel mutants exclusively generating long amyloid β-peptides. J. Biol. Chem. 280, 19070–19077 (2005).
Godbolt, A.K. et al. A presenilin 1 R278I mutation presenting with language impairment. Neurology 63, 1702–1704 (2004).
Shen, J. et al. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell 89, 629–639 (1997).
Wong, P.C. et al. Presenilin 1 is required for Notch1 and Dll1 expression in the paraxial mesoderm. Nature 387, 288–292 (1997).
Culvenor, J.G. et al. Characterization of presenilin complexes from mouse and human brain using blue native gel electrophoresis reveals high expression in embryonic brain and minimal change in complex mobility with pathogenic presenilin mutations. Eur. J. Biochem. 271, 375–385 (2003).
Evin, G. et al. Transition-state analogue γ-secretase inhibitors stabilize a 900 kDa presenilin/nicastrin complex. Biochemistry 44, 4332–4341 (2005).
Thinakaran, G. et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17, 181–190 (1996).
Lee, M.K. et al. Hyperaccumulation of FAD-linked presenilin 1 variants in vivo. Nat. Med. 3, 756–760 (1997).
Kaneko, H. et al. Enhanced accumulation of phosphorylated α-synuclein and elevated β-amyloid 42/40 ratio caused by expression of the presenilin-1 ΔT440 mutant associated with familial Lewy body disease and variant Alzheimer's disease. J. Neurosci. 27, 13092–13097 (2007).
Schroeter, E.H. et al. A presenilin dimer at the core of the γ-secretase enzyme: insight from parallel analysis of Notch1 and APP proteolysis. Proc. Natl. Acad. Sci. USA 100, 13075–13080 (2003).
Hama, E., Shirotani, K., Iwata, N. & Saido, T.C. Effects of neprilysin chimeric proteins targeted to subcellular compartments on amyloid β peptide clearance in primary neurons. J. Biol. Chem. 279, 30259–30264 (2004).
Kumar-Singh, S. et al. Mean age-of-onset of familial Alzheimer disease caused by presenilin mutations correlates with both increased Aβ42 and decreased Aβ40. Hum. Mutat. 27, 686–695 (2006).
Wang, R., Wang, B., He, W. & Zheng, H. Wild-type presenilin 1 protects against Alzheimer disease mutation–induced amyloid pathology. J. Biol. Chem. 281, 15330–15336 (2006).
Maeda, J. et al. Longitudinal, quantitative assessment of amyloid, neuroinflammation, and anti-amyloid treatment in a living mouse model of Alzheimer's disease enabled by positron emission tomography. J. Neurosci. 27, 10957–10968 (2007).
Saido, T.C. et al. Dominant and differential deposition of distinct β-amyloid peptide species, AßN3(pE), in senile plaques. Neuron 14, 457–466 (1995).
Schilling, S. et al. Glutaminyl cyclase inhibition attenuates pyroglutamate Aβ and Alzheimer's disease–like pathology. Nat. Med. 14, 1106–1111 (2008).
Zhang, C. et al. Presenilins are essential for regulating neurotransmitter release. Nature 460, 632–636 (2009).
Russo, C. et al. Presenilin-1 mutations in Alzheimer's disease. Nature 405, 531–532 (2000).
McGowan, E. et al. Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron 47, 191–199 (2005).
Kim, J. et al. Aβ40 inhibits amyloid deposition in vivo. J. Neurosci. 27, 627–633 (2007).
Ono, K., Condron, M. & Teplow, D.B. Effects of the English (H6R) and Tottori (D7N) familial Alzheimer disease mutations on amyloid β-protein assembly and toxicity. J. Biol. Chem. 285, 23186–23197 (2010).
Jan, A. et al. The ratio of monomeric to aggregated forms of Aβ40 and Aβ42 is an important determinant of amyloid-β aggregation, fibrillogenesis, and toxicity. J. Biol. Chem. 283, 28176–28189 (2008).
Huppert, S.S. et al. Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature 405, 966–970 (2000).
Ikeuchi, T. et al. Familial Alzheimer disease-linked presenilin 1 variants enhance production of both Aβ1–40 and Aβ1–42 peptides that are only partially sensitive to a potent aspartyl protease transition state inhibitor of “γ-secretase”. J. Biol. Chem. 278, 7010–7018 (2003).
Serneels, L. et al. γ-Secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer's disease. Science 324, 639–642 (2009).
Bentahir, M. et al. Presenilin clinical mutations can affect γ-secretase activity by different mechanisms. J. Neurochem. 96, 732–742 (2006).
Deng, Y. et al. Deletion of presenilin 1 hydrophilic loop sequence leads to imparired γ-secretase activity and exacerbated amyloid pathology. J. Neurosci. 26, 3845–3854 (2006).
Hara, H. et al. Development of a safe oral Aβ vaccine using recombinant adeno-associated virus vector for Alzheimer's disease. J. Alzheimers Dis. 6, 483–488 (2004).
Mouri, A. et al. Oral vaccination with a viral vector containing Aβ cDNA attenuates age-related Aβ accumulation and memory deficits without causing inflammation in a mouse Alzheimer model. FASEB J. 21, 2135–2148 (2007).
Kimura, N. et al. Senile plaques in an aged Western Lowland Gorilla. Exp. Anim. 50, 77–81 (2001).
Sturchler-Pierrat, C. et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease–like pathology. Proc. Natl. Acad. Sci. USA 94, 13287–13292 (1997).
Huang, S.-M. et al. Neprilysin-sensitive synapse-associated amyloid-β peptide oligomers impair neuronal plasticity and cognitive function. J. Biol. Chem. 281, 17941–17951 (2006).
Kopan, R., Schroeter, E.H., Weintraub, H. & Nye, J.S. Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proc. Natl. Acad. Sci. USA 93, 1683–1688 (1996).
Iwata, N. et al. Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-β peptide in mouse brain. J. Neurosci. 24, 991–998 (2004).
Iwatsubo, T. et al. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43). Neuron 13, 45–53 (1994).
Enya, M. et al. Appearance of sodium dodecylsulfate-stable amyloid β-protein (Aβ) dimer in the cortex during aging. Am. J. Pathol. 154, 271–279 (1999).
Ryan, D.A., Narrow, W.C., Federoff, H.J. & Bowers, W.J. An improved method for generating consistent soluble amyloid-beta oligomer preparations for in vitro neurotoxicity studies. J. Neurosci. Res. 190, 171–179 (2010).
Arango, D. et al. Systemic genetic study of Alzheimer disease in Latin America: mutation frequencies of the amyloid β precursor protein and presenilin gene in Colombia. Am. J. Med. Genet. 103, 138–143 (2001).
Acknowledgements
We thank M.N. Rossor (University College London) for sharing clinical information about the R278I mutation carriers, J.Q. Trojanowski and V.M.-Y. Lee (University of Pennsylvania) for providing postmortem brain tissues, R. Kopan (Washington University) for providing Myc-tagged ΔNotch1 plasmid, A. Takashima (RIKEN Brain Science Institute) for providing antibody to Aph-1, and J. Hardy (University College London) for valuable discussions. This work was supported by research grants from RIKEN Brain Science Institute, the Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Health, Labor and Welfare of Japan, the TAKEDA Science Foundation, the Fund for Scientific Research – Flanders (FWO-V), the Interuniversity Attraction Poles program P6/43 of the Belgian Federal Science Policy Office and a Methusalem Excellence Grant of the Flemish Government to C.V.B. N.B receives a FWO-V postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
This study was jointly designed by T. Saito, T. Suemoto and T.C.S. Experiments were performed by T. Saito, T. Suemoto, N.M., Y.M., K.Y. and S.F. T. Saito, T. Suemoto, S.F., K.Y., P.N., J.T., M.N., N.I., C.V.B., Y.I. and T.C.S. jointly analyzed and interpreted data. N.B., K.S. and C.V.B. identified pathogenic PS1 mutations in patients and families and generated PSEN1 vector constructs for expression studies.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–16 (PDF 6023 kb)
Rights and permissions
About this article
Cite this article
Saito, T., Suemoto, T., Brouwers, N. et al. Potent amyloidogenicity and pathogenicity of Aβ43. Nat Neurosci 14, 1023–1032 (2011). https://doi.org/10.1038/nn.2858
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2858
This article is cited by
-
Long-term oral administration of curcumin is effective in preventing short-term memory deterioration and prolonging lifespan in a mouse model of Alzheimer’s disease
Advances in Traditional Medicine (2024)
-
Amyloid β-based therapy for Alzheimer’s disease: challenges, successes and future
Signal Transduction and Targeted Therapy (2023)
-
Critical thinking of Alzheimer’s transgenic mouse model: current research and future perspective
Science China Life Sciences (2023)
-
Aβ profiles generated by Alzheimer’s disease causing PSEN1 variants determine the pathogenicity of the mutation and predict age at disease onset
Molecular Psychiatry (2022)
-
Somatostatin-evoked Aβ catabolism in the brain: Mechanistic involvement of α-endosulfine-KATP channel pathway
Molecular Psychiatry (2022)