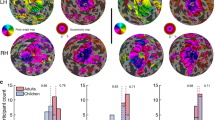
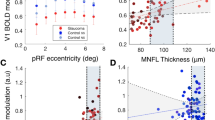
Abstract
The occipital lobe contains retinotopic representations of the visual field. The representation of the central retina in early visual areas (V1–3) is found at the occipital pole. When the central retina is lesioned in both eyes by macular degeneration, this region of visual cortex at the occipital pole is accordingly deprived of input. However, even when such lesions occur in adulthood, some visually driven activity in and around the occipital pole can be observed. It has been suggested that this activity is a result of remapping of this area so that it now responds to inputs from intact, peripheral retina. We evaluated whether or not remapping of visual cortex underlies this activity. Our functional magnetic resonance imaging results provide no evidence of remapping, questioning the contemporary view that early visual areas of the adult human brain have the capacity to reorganize extensively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Wandell, B.A., Dumoulin, S.O. & Brewer, A.A. Visual field maps in human cortex. Neuron 56, 366–383 (2007).
Morland, A.B., Baseler, H.A., Hoffmann, M.B., Sharpe, L.T. & Wandell, B.A. Abnormal retinotopic representations in human visual cortex revealed by fMRI. Acta Psychol. (Amst.) 107, 229–247 (2001).
Hoffmann, M.B., Tolhurst, D.J., Moore, A.T. & Morland, A.B. Organization of the visual cortex in human albinism. J. Neurosci. 23, 8921–8930 (2003).
Muckli, L., Naumer, M.J. & Singer, W. Bilateral visual field maps in a patient with only one hemisphere. Proc. Natl. Acad. Sci. USA 106, 13034–13039 (2009).
Levin, N., Dumoulin, S.O., Winawer, J., Dougherty, R.F. & Wandell, B.A. Cortical maps and white matter tracts following long period of visual deprivation and retinal image restoration. Neuron 65, 21–31 (2010).
Baseler, H.A. et al. Reorganization of human cortical maps caused by inherited photoreceptor abnormalities. Nat. Neurosci. 5, 364–370 (2002).
Kaas, J.H. et al. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science 248, 229–231 (1990).
Heinen, S.J. & Skavenski, A.A. Recovery of visual responses in foveal V1 neurons following bilateral foveal lesions in adult monkey. Exp. Brain Res. 83, 670–674 (1991).
Chino, Y.M., Kaas, J.H., Smith, E.L. III, Langston, A.L. & Cheng, H. Rapid reorganization of cortical maps in adult cats following restricted deafferentation in retina. Vision Res. 32, 789–796 (1992).
Gilbert, C.D. & Wiesel, T.N. Receptive field dynamics in adult primary visual cortex. Nature 356, 150–152 (1992).
Darian-Smith, C. & Gilbert, C.D. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J. Neurosci. 15, 1631–1647 (1995).
Kaas, J.H. Sensory loss and cortical reorganization in mature primates. Prog. Brain Res. 138, 167–176 (2002).
Giannikopoulos, D.V. & Eysel, U.T. Dynamics and specificity of cortical map reorganization after retinal lesions. Proc. Natl. Acad. Sci. USA 103, 10805–10810 (2006).
Sunness, J.S., Liu, T. & Yantis, S. Retinotopic mapping of the visual cortex using functional magnetic resonance imaging in a patient with central scotomas from atrophic macular degeneration. Ophthalmology 111, 1595–1598 (2004).
Baker, C.I., Peli, E., Knouf, N. & Kanwisher, N.G. Reorganization of visual processing in macular degeneration. J. Neurosci. 25, 614–618 (2005).
Schumacher, E.H. et al. Reorganization of visual processing is related to eccentric viewing in patients with macular degeneration. Restor. Neurol. Neurosci. 26, 391–402 (2008).
Cowey, A. & Walsh, V. Magnetically induced phosphenes in sighted, blind and blindsighted observers. Neuroreport 11, 3269–3273 (2000).
Masuda, Y., Dumoulin, S.O., Nakadomari, S. & Wandell, B.A. V1 projection zone signals in human macular degeneration depend on task, not stimulus. Cereb. Cortex 18, 2483–2493 (2008).
Wandell, B.A. & Smirnakis, S.M. Plasticity and stability of visual field maps in adult primary visual cortex. Nat. Rev. Neurosci. 10, 873–884 (2009).
Masuda, Y. et al. Task-dependent V1 responses in human retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 51, 5356–5364 (2010).
DeYoe, E.A. et al. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc. Natl. Acad. Sci. USA 93, 2382–2386 (1996).
Engel, S.A., Glover, G.H. & Wandell, B.A. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb. Cortex 7, 181–192 (1997).
Engel, S.A. et al. fMri of human visual cortex. Nature 369, 525 (1994).
Sereno, M.I. et al. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268, 889–893 (1995).
Smirnakis, S.M. et al. Lack of long-term cortical reorganization after macaque retinal lesions. Nature 435, 300–307 (2005).
Baker, C.I., Dilks, D.D., Peli, E. & Kanwisher, N. Reorganization of visual processing in macular degeneration: replication and clues about the role of foveal loss. Vision Res. 48, 1910–1919 (2008).
Crossland, M.D., Morland, A.B., Feely, M.P., von dem Hagen, E. & Rubin, G.S. The effect of age and fixation instability on retinotopic mapping of primary visual cortex. Invest. Ophthalmol. Vis. Sci. 49, 3734–3739 (2008).
Parrish, T.B., Gitelman, D.R., LaBar, K.S. & Mesulam, M.M. Impact of signal-to-noise on functional MRI. Magn. Reson. Med. 44, 925–932 (2000).
Cavanaugh, J.R., Bair, W. & Movshon, J.A. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. J. Neurophysiol. 88, 2530–2546 (2002).
Dumoulin, S.O. & Wandell, B.A. Population receptive field estimates in human visual cortex. Neuroimage 39, 647–660 (2008).
Andrews, T.J., Halpern, S.D. & Purves, D. Correlated size variations in human visual cortex, lateral geniculate nucleus, and optic tract. J. Neurosci. 17, 2859–2868 (1997).
Hubel, D.H. & Wiesel, T.N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. (Lond.) 206, 419–436 (1970).
Hubel, D.H., Wiesel, T.N. & LeVay, S. Plasticity of ocular dominance columns in monkey striate cortex. Phil. Trans. R. Soc. Lond. B 278, 377–409 (1977).
Le Vay, S., Wiesel, T.N. & Hubel, D.H. The development of ocular dominance columns in normal and visually deprived monkeys. J. Comp. Neurol. 191, 1–51 (1980).
Horton, J.C. & Hocking, D.R. Timing of the critical period for plasticity of ocular dominance columns in macaque striate cortex. J. Neurosci. 17, 3684–3709 (1997).
Williams, M.A. et al. Feedback of visual object information to foveal retinotopic cortex. Nat. Neurosci. 11, 1439–1445 (2008).
Angelucci, A. & Bullier, J. Reaching beyond the classical receptive field of V1 neurons: horizontal or feedback axons? J. Physiol. (Paris) 97, 141–154 (2003).
Angelucci, A. & Sainsbury, K. Contribution of feedforward thalamic afferents and corticogeniculate feedback to the spatial summation area of macaque V1 and LGN. J. Comp. Neurol. 498, 330–351 (2006).
Lund, J.S. Anatomical organization of macaque monkey striate visual cortex. Annu. Rev. Neurosci. 11, 253–288 (1988).
Gilbert, C.D. & Wiesel, T.N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature 280, 120–125 (1979).
Dougherty, R.F. et al. Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J. Vis. 3, 586–598 (2003).
Horton, J.C. & Hocking, D.R. Monocular core zones and binocular border strips in primate striate cortex revealed by the contrasting effects of enucleation, eyelid suture, and retinal laser lesions on cytochrome oxidase activity. J. Neurosci. 18, 5433–5455 (1998).
Liu, T. et al. Incomplete cortical reorganization in macular degeneration. Invest. Ophthalmol. Vis. Sci. 51, 6826–6834 (2010).
Boucard, C.C. et al. Changes in cortical grey matter density associated with long-standing retinal visual field defects. Brain 132, 1898–1906 (2009).
Teo, P.C., Sapiro, G. & Wandell, B.A. Creating connected representations of cortical gray matter for functional MRI visualization. IEEE Trans. Med. Imaging 16, 852–863 (1997).
Wandell, B.A., Chial, S. & Backus, B.T. Visualization and measurement of the cortical surface. J. Cogn. Neurosci. 12, 739–752 (2000).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002).
Wandell, B.A., Brewer, A.A. & Dougherty, R.F. Visual field map clusters in human cortex. Phil. Trans. R. Soc. Lond. B 360, 693–707 (2005).
Lewis, S.M. et al. Logarithmic transformation for high-field BOLD fMRI data. Exp. Brain Res. 165, 447–453 (2005).
Winawer, J., Horiguchi, H., Sayres, R.A., Amano, K. & Wandell, B.A. Mapping hV4 and ventral occipital cortex: the venous eclipse. J. Vis. 10, 1–22 (2010).
Acknowledgements
We would like to thank all of our participants. We thank Edward Silson for constructive discussion of the manuscript. We are also grateful to the Medical Research Council for funding this study (G0401339). K.V.H. and F.W.C. were supported by a grant from Stichting Nederlands Oogheelkundig Onderzoek and by European Union grants #043157 (Syntex) and #043261 (Percept). A.T., G.S.R. and M.D.C. also received financial support from the Department of Health through an award made by the National Institute for Health Research to Moorfields Eye Hospital National Health Service (NHS) Foundation Trust and University College London Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, the Department of Health or the EU commission.
Author information
Authors and Affiliations
Contributions
H.A.B. and A.G. acquired and analyzed the neuroimaging data and prepared the manuscript. K.V.H. designed and implemented an analysis to determine the population receptive field characteristics and prepared the manuscript. C.R. acquired neuroimaging data. M.D.C. recruited patients, acquired and analyzed clinical data. A.T. recruited and assessed patients. G.S.R. jointly designed the study, recruited patients and acquired and analyzed clinical data. F.W.C. designed an analysis to determine the population receptive field characteristics and prepared the manuscript. A.B.M. jointly designed the study, acquired and analyzed the neuroimaging data and prepared the manuscript. All authors contributed to drafts of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 and Supplementary Results (PDF 154 kb)
Rights and permissions
About this article
Cite this article
Baseler, H., Gouws, A., Haak, K. et al. Large-scale remapping of visual cortex is absent in adult humans with macular degeneration. Nat Neurosci 14, 649–655 (2011). https://doi.org/10.1038/nn.2793
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2793
This article is cited by
-
Assessing the structure of the posterior visual pathway in bilateral macular degeneration
Scientific Reports (2023)
-
Local neuroplasticity in adult glaucomatous visual cortex
Scientific Reports (2022)
-
Assessing functional reorganization in visual cortex with simulated retinal lesions
Brain Structure and Function (2021)
-
Contextual influences in the peripheral retina of patients with macular degeneration
Scientific Reports (2019)
-
Plasticity versus stability across the human cortical visual connectome
Nature Communications (2019)