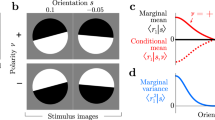
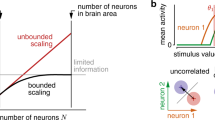
Abstract
Visual function depends on the accuracy of signals carried by visual cortical neurons. Combining information across neurons should improve this accuracy because single neuron activity is variable. We examined the reliability of information inferred from populations of simultaneously recorded neurons in macaque primary visual cortex. We considered a decoding framework that computes the likelihood of visual stimuli from a pattern of population activity by linearly combining neuronal responses and tested this framework for orientation estimation and discrimination. We derived a simple parametric decoder assuming neuronal independence and a more sophisticated empirical decoder that learned the structure of the measured neuronal response distributions, including their correlated variability. The empirical decoder used the structure of these response distributions to perform better than its parametric variant, indicating that their structure contains critical information for sensory decoding. These results show how neuronal responses can best be used to inform perceptual decision-making.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
16 January 2011
In the version of this article initially published online, an error was made in the legend for Figure 6. In the legend, 0.75% should read 0.75. This error has been corrected for the print, PDF and HTML versions of this article.
References
Pouget, A., Dayan, P. & Zemel, R.S. Inference and computation with population codes. Annu. Rev. Neurosci. 26, 381–410 (2003).
Averbeck, B.B., Latham, P.E. & Pouget, A. Neural correlations, population coding and computation. Nat. Rev. Neurosci. 7, 358–366 (2006).
Newsome, W.T., Britten, K.H. & Movshon, J.A. Neuronal correlates of a perceptual decision. Nature 341, 52–54 (1989).
Oram, M.W., Foldiak, P., Perrett, D.I. & Sengpiel, F. The 'Ideal Homunculus': decoding neural population signals. Trends Neurosci. 21, 259–265 (1998).
Sanger, T.D. Probability density estimation for the interpretation of neural population codes. J. Neurophysiol. 76, 2790–2793 (1996).
Foldiak, P. The “Ideal Homunculus”: statistical inference from neural population responses. in Computation and Neural Systems (eds. Eeckman, F.H. & Bower, J.M.) 55–60 (Kluwer Academic, 1993).
Bradley, A., Skottun, B.C., Ohzawa, I., Sclar, G. & Freeman, R.D. Visual orientation and spatial frequency discrimination: a comparison of single neurons and behavior. J. Neurophysiol. 57, 755–772 (1987).
Geisler, W.S. & Albrecht, D.G. Visual cortex neurons in monkeys and cats: detection, discrimination, and identification. Vis. Neurosci. 14, 897–919 (1997).
Tolhurst, D.J., Movshon, J.A. & Dean, A.F. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res. 23, 775–785 (1983).
Vogels, R. & Orban, G.A. How well do response changes of striate neurons signal differences in orientation: a study in the discriminating monkey. J. Neurosci. 10, 3543–3558 (1990).
Braitenberg, V. & Schüz, A. Cortex: Statistics and Geometry of Neuronal Connectivity (Springer Verlag, 1998).
Britten, K.H., Newsome, W.T., Shadlen, M.N., Celebrini, S. & Movshon, J.A. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis. Neurosci. 13, 87–100 (1996).
Shadlen, M.N., Britten, K.H., Newsome, W.T. & Movshon, J.A. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J. Neurosci. 16, 1486–1510 (1996).
Abbott, L.F. & Dayan, P. The effect of correlated variability on the accuracy of a population code. Neural Comput. 11, 91–101 (1999).
Butts, D.A. & Goldman, M.S. Tuning curves, neuronal variability, and sensory coding. PLoS Biol. 4, e92 (2006).
Smith, M.A. & Kohn, A. Spatial and temporal scales of neuronal correlation in primary visual cortex. J. Neurosci. 28, 12591–12603 (2008).
Montani, F., Kohn, A., Smith, M.A. & Schultz, S.R. The role of correlations in direction and contrast coding in the primary visual cortex. J. Neurosci. 27, 2338–2348 (2007).
Zohary, E., Shadlen, M.N. & Newsome, W.T. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature 370, 140–143 (1994).
Deneve, S., Latham, P.E. & Pouget, A. Reading population codes: a neural implementation of ideal observers. Nat. Neurosci. 2, 740–745 (1999).
Shamir, M. & Sompolinsky, H. Implications of neuronal diversity on population coding. Neural Comput. 18, 1951–1986 (2006).
Georgopoulos, A.P., Schwartz, A.B. & Kettner, R.E. Neuronal population coding of movement direction. Science 233, 1416–1419 (1986).
Salinas, E. & Abbott, L.F. Vector reconstruction from firing rates. J. Comput. Neurosci. 1, 89–107 (1994).
Dayan, P. & Abbott, L.F. Theoretical Neuroscience (MIT Press, 2001).
Seriès, P., Latham, P.E. & Pouget, A. Tuning curve sharpening for orientation selectivity: coding efficiency and the impact of correlations. Nat. Neurosci. 7, 1129–1135 (2004).
Jazayeri, M. & Movshon, J.A. Optimal representation of sensory information by neural populations. Nat. Neurosci. 9, 690–696 (2006).
Ma, W.J., Beck, J.M., Latham, P.E. & Pouget, A. Bayesian inference with probabilistic population codes. Nat. Neurosci. 9, 1432–1438 (2006).
Jaynes, E.T. Probability Theory: The Logic of Science (Cambridge University Press, 2003).
Seung, H.S. & Sompolinsky, H. Simple models for reading neuronal population codes. Proc. Natl. Acad. Sci. USA 90, 10749–10753 (1993).
Simoncelli, E. Optimal estimation in sensory systems. in The Cognitive Neurosciences (MIT Press, 2009).
Beverley, K.I. & Regan, D. The relation between discrimination and sensitivity in the perception of motion in depth. J. Physiol. (Lond.) 249, 387–398 (1975).
Jazayeri, M. & Movshon, J.A. A new perceptual illusion reveals mechanisms of sensory decoding. Nature 446, 912–915 (2007).
Ernst, M.O. & Banks, M.S. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415, 429–433 (2002).
Tassinari, H., Hudson, T.E. & Landy, M.S. Combining priors and noisy visual cues in a rapid pointing task. J. Neurosci. 26, 10154–10163 (2006).
Yang, T. & Shadlen, M.N. Probabilistic reasoning by neurons. Nature 447, 1075–1080 (2007).
Zhang, K., Ginzburg, I., McNaughton, B.L. & Sejnowski, T.J. Interpreting neuronal population activity by reconstruction: unified framework with application to hippocampal place cells. J. Neurophysiol. 79, 1017–1044 (1998).
Averbeck, B.B. & Lee, D. Effects of noise correlations on information encoding and decoding. J. Neurophysiol. 95, 3633–3644 (2006).
Benucci, A., Ringach, D.L. & Carandini, M. Coding of stimulus sequences by population responses in visual cortex. Nat. Neurosci. 12, 1317–1324 (2009).
Romo, R., Hernandez, A., Zainos, A. & Salinas, E. Correlated neuronal discharges that increase coding efficiency during perceptual discrimination. Neuron 38, 649–657 (2003).
Pillow, J.W. et al. Spatio-temporal correlations and visual signaling in a complete neuronal population. Nature 454, 995–999 (2008).
Gutnisky, D.A. & Dragoi, V. Adaptive coding of visual information in neural populations. Nature 452, 220–224 (2008).
Wu, S., Nakahara, H. & Amari, S. Population coding with correlation and an unfaithful model. Neural Comput. 13, 775–797 (2001).
Nirenberg, S., Carcieri, S.M., Jacobs, A.L. & Latham, P.E. Retinal ganglion cells act largely as independent encoders. Nature 411, 698–701 (2001).
Beck, J.M. et al. Probabilistic population codes for Bayesian decision making. Neuron 60, 1142–1152 (2008).
Schölkopf, B. & Smola, A. Learning with Kernels (MIT Press, 2002).
Vapnik, V. The Nature of Statistical Learning Theory (Springer, 2000).
Ecker, A.S. et al. Decorrelated neuronal firing in cortical microcircuits. Science 327, 584–587 (2010).
Carandini, M., Heeger, D.J. & Movshon, J.A. Linearity and normalization in simple cells of the macaque primary visual cortex. J. Neurosci. 17, 8621–8644 (1997).
Law, C.T. & Gold, J.I. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat. Neurosci. 11, 505–513 (2008).
Bair, W., Cavanaugh, J.R., Smith, M.A. & Movshon, J.A. The timing of response onset and offset in macaque visual neurons. J. Neurosci. 22, 3189–3205 (2002).
Schmolesky, M.T. et al. Signal timing across the macaque visual system. J. Neurophysiol. 79, 3272–3278 (1998).
Acknowledgements
We are grateful to M. Smith and R. Kelly for their help with recording and to E. Simoncelli and M. Yanike for helpful comments on the manuscript. This research was supported by US National Institutes of Health research grants EY2017 and EY4440, training grant EY7158 and the Swartz Foundation.
Author information
Authors and Affiliations
Contributions
A.B.A.G., A.K. and J.A.M. designed the experiments, A.B.A.G. and A.K. collected the data, A.B.A.G. created the models and analyzed the data, A.B.A.G. and J.A.M. wrote the manuscript, and A.K. and M.J. contributed to the intellectual development of the project and to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 2190 kb)
Rights and permissions
About this article
Cite this article
Graf, A., Kohn, A., Jazayeri, M. et al. Decoding the activity of neuronal populations in macaque primary visual cortex. Nat Neurosci 14, 239–245 (2011). https://doi.org/10.1038/nn.2733
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2733
This article is cited by
-
Category representation in primary visual cortex after visual perceptual learning
Cognitive Neurodynamics (2024)
-
Retinal spike train decoder using vector quantization for visual scene reconstruction
Complex & Intelligent Systems (2024)
-
Bayesian encoding and decoding as distinct perspectives on neural coding
Nature Neuroscience (2023)
-
The structures and functions of correlations in neural population codes
Nature Reviews Neuroscience (2022)
-
Neural tuning and representational geometry
Nature Reviews Neuroscience (2021)