Abstract
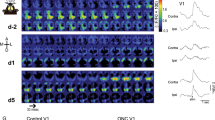
Changes in visual cortical responses that are induced by monocular visual deprivation are a widely studied example of competitive, experience-dependent neural plasticity. It has been thought that the deprived-eye pathway will fail to compete against the open-eye pathway for limited amounts of brain-derived neurotrophic factor, which acts on TrkB and is needed to sustain effective synaptic connections. We tested this model by using a chemical-genetic approach in mice to inhibit TrkB kinase activity rapidly and specifically during the induction of cortical plasticity in vivo. Contrary to the model, TrkB kinase activity was not required for any of the effects of monocular deprivation. When the deprived eye was re-opened during the critical period, cortical responses to it recovered. This recovery was blocked by TrkB inhibition. These findings suggest a more conventional trophic role for TrkB signaling in the enhancement of responses or growth of new connections, rather than a role in competition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wiesel, T.N. Postnatal development of the visual cortex and the influence of environment. Nature 299, 583–591 (1982).
Reiter, H.O., Waitzman, D.M. & Stryker, M.P. Cortical activity blockade prevents ocular dominance plasticity in the kitten visual cortex. Exp. Brain Res. 65, 182–188 (1986).
Reiter, H.O. & Stryker, M.P. Neural plasticity without postsynaptic action potentials: less-active inputs become dominant when kitten visual cortical cells are pharmacologically inhibited. Proc. Natl. Acad. Sci. USA 85, 3623–3627 (1988).
Hata, Y. & Stryker, M.P. Control of thalamocortical afferent rearrangement by postsynaptic activity in developing visual cortex. Science 265, 1732–1735 (1994).
Hata, Y., Tsumoto, T. & Stryker, M.P. Selective pruning of more active afferents when cat visual cortex is pharmacologically inhibited. Neuron 22, 375–381 (1999).
Lessmann, V., Gottmann, K. & Malcangio, M. Neurotrophin secretion: current facts and future prospects. Prog. Neurobiol. 69, 341–374 (2003).
Lu, B. BDNF and activity-dependent synaptic modulation. Learn. Mem. 10, 86–98 (2003).
McAllister, A.K., Katz, L.C. & Lo, D.C. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 22, 295–318 (1999).
Poo, M.M. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2, 24–32 (2001).
Castren, E., Zafra, F., Thoenen, H. & Lindholm, D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc. Natl. Acad. Sci. USA 89, 9444–9448 (1992).
Bozzi, Y. et al. Monocular deprivation decreases the expression of messenger RNA for brain-derived neurotrophic factor in the rat visual cortex. Neuroscience 69, 1133–1144 (1995).
Schoups, A.A., Elliott, R.C., Friedman, W.J. & Black, I.B. NGF and BDNF are differentially modulated by visual experience in the developing geniculocortical pathway. Brain Res. Dev. Brain Res. 86, 326–334 (1995).
Majdan, M. & Shatz, C.J. Effects of visual experience on activity-dependent gene regulation in cortex. Nat. Neurosci. 9, 650–659 (2006).
Galuske, R.A., Kim, D.S., Castren, E. & Singer, W. Differential effects of neurotrophins on ocular dominance plasticity in developing and adult cat visual cortex. Eur. J. Neurosci. 12, 3315–3330 (2000).
Gillespie, D.C., Crair, M.C. & Stryker, M.P. Neurotrophin-4/5 alters responses and blocks the effect of monocular deprivation in cat visual cortex during the critical period. J. Neurosci. 20, 9174–9186 (2000).
Lodovichi, C., Berardi, N., Pizzorusso, T. & Maffei, L. Effects of neurotrophins on cortical plasticity: same or different? J. Neurosci. 20, 2155–2165 (2000).
Shatz, C.J. Neurotrophins and visual system plasticity. in Molecular and Cellular Approaches to Neural Development (eds. Cowan, M.W., Jessell, T.M. & Zipursky, S.L.) 509–524 (Oxford University Press, New York, 1997).
Hanover, J.L., Huang, Z.J., Tonegawa, S. & Stryker, M.P. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J. Neurosci. 19, RC40 (1999).
Huang, Z.J. et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755 (1999).
Thoenen, H. Neurotrophins and neuronal plasticity. Science 270, 593–598 (1995).
Bonhoeffer, T. Neurotrophins and activity-dependent development of the neocortex. Curr. Opin. Neurobiol. 6, 119–126 (1996).
Hensch, T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888 (2005).
Specht, K.M. & Shokat, K.M. The emerging power of chemical genetics. Curr. Opin. Cell Biol. 14, 155–159 (2002).
Chen, X. et al. A chemical-genetic approach to studying neurotrophin signaling. Neuron 46, 13–21 (2005).
Wang, H. et al. Inducible protein knockout reveals temporal requirement of CaMKII reactivation for memory consolidation in the brain. Proc. Natl. Acad. Sci. USA 100, 4287–4292 (2003).
Hensch, T.K. et al. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282, 1504–1508 (1998).
Cang, J., Kalatsky, V.A., Lowel, S. & Stryker, M.P. Optical imaging of the intrinsic signal as a measure of cortical plasticity in the mouse. Vis. Neurosci. 22, 685–691 (2005).
Mioche, L. & Singer, W. Chronic recordings from single sites of kitten striate cortex during experience-dependent modifications of receptive-field properties. J. Neurophysiol. 62, 185–197 (1989).
Frenkel, M.Y. & Bear, M.F. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron 44, 917–923 (2004).
Bear, M.F., Kleinschmidt, A., Gu, Q.A. & Singer, W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J. Neurosci. 10, 909–925 (1990).
Liao, D.S., Mower, A.F., Neve, R.L., Sato-Bigbee, C. & Ramoa, A.S. Different mechanisms for loss and recovery of binocularity in the visual cortex. J. Neurosci. 22, 9015–9023 (2002).
Krahe, T.E., Medina, A.E., de Bittencourt-Navarrete, R.E., Colello, R.J. & Ramoa, A.S. Protein synthesis–independent plasticity mediates rapid and precise recovery of deprived eye responses. Neuron 48, 329–343 (2005).
Ugolini, G., Cremisi, F. & Maffei, L. TrkA, TrkB and p75 mRNA expression is developmentally regulated in the rat retina. Brain Res. 704, 121–124 (1995).
Rutherford, L.C., Nelson, S.B. & Turrigiano, G.G. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron 21, 521–530 (1998).
Vicario-Abejon, C., Owens, D., McKay, R. & Segal, M. Role of neurotrophins in central synapse formation and stabilization. Nat. Rev. Neurosci. 3, 965–974 (2002).
Lu, B., Pang, P.T. & Woo, N.H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 6, 603–614 (2005).
Reichardt, L.F. Neurotrophin-regulated signaling pathways. Phil. Trans. R. Soc. Lond. B 361, 1545–1564 (2006).
Minichiello, L. et al. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron 36, 121–137 (2002).
Korte, M., Minichiello, L., Klein, R. & Bonhoeffer, T. Shc-binding site in the TrkB receptor is not required for hippocampal long-term potentiation. Neuropharmacology 39, 717–724 (2000).
Lessmann, V. & Heumann, R. Modulation of unitary glutamatergic synapses by neurotrophin-4/5 or brain-derived neurotrophic factor in hippocampal microcultures: presynaptic enhancement depends on pre-established paired-pulse facilitation. Neuroscience 86, 399–413 (1998).
Schinder, A.F., Berninger, B. & Poo, M. Postsynaptic target specificity of neurotrophin-induced presynaptic potentiation. Neuron 25, 151–163 (2000).
Tyler, W.J. et al. BDNF increases release probability and the size of a rapidly recycling vesicle pool within rat hippocampal excitatory synapses. J. Physiol. (Lond.) 574, 787–803 (2006).
Li, Y.X., Zhang, Y., Lester, H.A., Schuman, E.M. & Davidson, N. Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. J. Neurosci. 18, 10231–10240 (1998).
Kang, H. & Schuman, E.M. Intracellular Ca2+ signaling is required for neurotrophin-induced potentiation in the adult rat hippocampus. Neurosci. Lett. 282, 141–144 (2000).
Blum, R. & Konnerth, A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology (Bethesda) 20, 70–78 (2005).
Gordon, J.A. & Stryker, M.P. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J. Neurosci. 16, 3274–3286 (1996).
Taha, S. & Stryker, M.P. Rapid ocular dominance plasticity requires cortical but not geniculate protein synthesis. Neuron 34, 425–436 (2002).
Aloyz, R., Fawcett, J.P., Kaplan, D.R., Murphy, R.A. & Miller, F.D. Activity-dependent activation of TrkB neurotrophin receptors in the adult CNS. Learn. Mem. 6, 216–231 (1999).
Kalatsky, V.A. & Stryker, M.P. New paradigm for optical imaging: temporally encoded maps of intrinsic signal. Neuron 38, 529–545 (2003).
Cang, J. et al. Ephrin-as guide the formation of functional maps in the visual cortex. Neuron 48, 577–589 (2005).
Acknowledgements
The TrkBF616A mice were provided by D. Ginty and with the permission of CGI and Princeton University. We thank members of the laboratory and P. McQuillen for discussions, J. Cang for help with analyses of optical imaging data, A. Shreiber, C.M. Beal and T. Tran for technical assistance, Regeneron Pharmaceuticals for TrkB-IgG, and L. Reichardt for TrkB antibody. This study was supported by grants from the US National Institutes of Health (P50-MH077972, R37-EY02874, and T32-EY07120).
Author information
Authors and Affiliations
Contributions
M.K. carried out all of the biochemical analysis, optical imaging and single-unit recordings using TrkBF616A and their appropriate control mice, prepared all of the figures (except for Supplementary Fig. 3) and wrote the first draft of the manuscript. J.L.H. carried out experiments on K252a and TrkB-IgG mice. P.M.E. supplied mutant mice and 1NM-PP1. M.K., P.M.E. and M.P.S. designed the experiments and assisted with data analysis and revision of the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 6447 kb)
Rights and permissions
About this article
Cite this article
Kaneko, M., Hanover, J., England, P. et al. TrkB kinase is required for recovery, but not loss, of cortical responses following monocular deprivation. Nat Neurosci 11, 497–504 (2008). https://doi.org/10.1038/nn2068
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn2068
This article is cited by
-
Dendrites help mitigate the plasticity-stability dilemma
Scientific Reports (2023)
-
The antidepressant fluoxetine acts on energy balance and leptin sensitivity via BDNF
Scientific Reports (2018)
-
Monocular deprivation induces dendritic spine elimination in the developing mouse visual cortex
Scientific Reports (2017)
-
Activity in motor–sensory projections reveals distributed coding in somatosensation
Nature (2012)
-
Nurturing brain plasticity: impact of environmental enrichment
Cell Death & Differentiation (2010)