Abstract
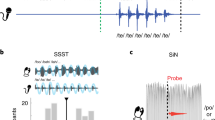
Speech production, like other sensorimotor behaviors, relies on multiple sensory inputs—audition, proprioceptive inputs from muscle spindles and cutaneous inputs from mechanoreceptors in the skin and soft tissues of the vocal tract. However, the capacity for intelligible speech by deaf speakers suggests that somatosensory input alone may contribute to speech motor control and perhaps even to speech learning. We assessed speech motor learning in cochlear implant recipients who were tested with their implants turned off. A robotic device was used to alter somatosensory feedback by displacing the jaw during speech. We found that implant subjects progressively adapted to the mechanical perturbation with training. Moreover, the corrections that we observed were for movement deviations that were exceedingly small, on the order of millimeters, indicating that speakers have precise somatosensory expectations. Speech motor learning is substantially dependent on somatosensory input.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cowie, R., Douglas-Cowie, R.E. & Kerr, A.G. A study of speech deterioration in post-lingually deafened adults. J. Laryngol. Otol. 96, 101–112 (1982).
Waldstein, R.S. Effects of post-lingual deafness on speech production: implications for the role of auditory feedback. J. Acoust. Soc. Am. 88, 2099–2114 (1990).
Perkell, J.S. et al. Time course of speech changes in response to unanticipated short-term changes in hearing state. J. Acoust. Soc. Am. 121, 505–518 (2007).
Hamlet, S.L. & Stone, M.L. Compensatory vowel characteristics resulting from the presence of different types of experimental dental protheses. J. Phon. 4, 199–218 (1976).
Lindblom, B., Lubker, J. & Gay, T. Formant frequencies of some xed-mandible vowels and a model of speech motor programming by predictive simulation. J. Phon. 7, 147–161 (1979).
Abbs, J.H. & Gracco, V.L. Sensorimotor actions in the control of multi-movement speech gestures. Trends Neurosci. 6, 391–395 (1983).
McFarland, D.H. & Baum, S. Incomplete compensation to articulatory perturbation. J. Acoust. Soc. Am. 97, 1865–1873 (1995).
Savariaux, C., Perrier, P. & Orliaguet, J.P. Compensation strategies for the perturbation of the rounded vowel [u] using a lip tube: A study of the control of space in speech production. J. Acoust. Soc. Am. 98, 2428–2442 (1995).
McFarland, D.H., Baum, S.R. & Chabot, C. Speech compensation to structural modifications of the oral cavity. J. Acoust. Soc. Am. 100, 1093–1104 (1996).
Aasland, W.A., Baum, S.R. & McFarland, D.H. Electropalatographic, acoustic and perceptual data on adaptation to a palatal perturbation. J. Acoust. Soc. Am. 119, 2372–2381 (2006).
Tremblay, S., Shiller, D.M. & Ostry, D.J. Somatosensory basis of speech production. Nature 423, 866–869 (2003).
Nasir, S.M. & Ostry, D.J. Somatosensory precision in speech production. Curr. Biol. 16, 1918–1923 (2006).
Houde, J.F. & Jordan, F.M. Sensorimotor adaptation in speech production. Science 279, 1213–1216 (1998).
Jones, J.A. & Munhall, K.G. Remapping auditory-motor representations in voice production. Curr. Biol. 15, 1768–1772 (2005).
Villacorta, V.M., Perkell, J.S. & Guenther, F.H. Sensorimotor adaptation to feedback perturbations of vowel acoustics and its relation to perception. J. Acoust. Soc. Am. 122, 2306–2319 (2007).
Guenther, F.H., Ghosh, S.S. & Tourville, J.A. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang. 96, 280–301 (2006).
Lackner, J.R. & Dizio, P. Rapid adaptation to coriolis force perturbations of arm trajectory. J. Neurophysiol. 72, 299–313 (1994).
Shadmehr, R. & Mussa-Ivaldi, F.A. Adaptive representation of dynamics during learning of a motor task. J. Neurosci. 14, 3208–3224 (1994).
Purcell, D.W. & Munhall, K.G. Adaptive control of vowel formant frequency: evidence from real-time formant manipulation. J. Acoust. Soc. Am. 119, 2288–2297 (2006).
Kapteyn, T.S., Boezeman, E.H.J.F. & Snel, A.M. Bone-conduction measurement and calibration using the cancellation method. J. Acoust. Soc. Am. 74, 1297–1299 (1983).
Purcell, D., Kunov, H. & Cleghorn, W. Objective calibration of bone conductors using otoacoustic emissions. Ear Hear. 20, 375–392 (1999).
Pörschmann, C. Influences of bone conduction and air conduction on the sound of one's own voice. Acta Acust. 86, 1038–1045 (2000).
Ernst, M.O. & Banks, M.S. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415, 429–433 (2002).
Sober, S.J. & Sabes, P.N. Multisensory integration during motor planning. J. Neurosci. 23, 6982–6992 (2003).
Svirsky, M.A. & Tobey, E.A. Effect of different types of auditory stimulation on vowel formant frequencies in multichannel cochlear implant users. J. Acoust. Soc. Am. 89, 2895–2904 (1991).
Svirsky, M.A., Lane, H., Perkell, J.S. & Wozniak, J. Effects of short-term auditory deprivation on speech production in adult cochlear implant users. J. Acoust. Soc. Am. 92, 1284–1300 (1992).
Acknowledgements
The authors thank L. Polka, D. Purcell, D. Shiller and M. Tiede for advice and assistance with auditory testing. This research was supported by US National Institute on Deafness and Other Communication Disorders grant DC-04669, the Natural Sciences and Engineering Research Council (Canada) and Fonds Québécois de la Recherche sur la Nature et les Technologies (Québec).
Author information
Authors and Affiliations
Contributions
S.M.N. and D.J.O. designed the experiments and wrote the manuscript. S.M.N. conducted the experiments and analyzed the data.
Corresponding author
Rights and permissions
About this article
Cite this article
Nasir, S., Ostry, D. Speech motor learning in profoundly deaf adults. Nat Neurosci 11, 1217–1222 (2008). https://doi.org/10.1038/nn.2193
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2193
This article is cited by
-
The functional relations among motor-based prediction, sensory goals and feedback in learning non-native speech sounds: Evidence from adult Mandarin Chinese speakers with an auditory feedback masking paradigm
Scientific Reports (2018)
-
Neural bases of sensorimotor adaptation in the vocal motor system
Experimental Brain Research (2018)
-
Temporal and spectral audiotactile interactions in musicians
Experimental Brain Research (2017)
-
New Directions for Understanding Neural Control in Swallowing: The Potential and Promise of Motor Learning
Dysphagia (2013)
-
A model for production, perception, and acquisition of actions in face-to-face communication
Cognitive Processing (2010)