Abstract
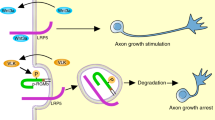
To define the role of the Raf serine/threonine kinases in nervous system development, we conditionally targeted B-Raf and C-Raf, two of the three known mammalian Raf homologs, using a mouse line expressing Cre recombinase driven by a nestin promoter. Targeting of B-Raf, but not C-Raf, markedly attenuated baseline phosphorylation of Erk in neural tissues and led to growth retardation. Conditional elimination of B-Raf in dorsal root ganglion (DRG) neurons did not interfere with survival, but instead caused marked eduction in expression of the glial cell line–derived neurotrophic factor receptor Ret at postnatal stages, associated with a profound reduction in levels of transcription factor CBF-β. Elimination of both alleles of Braf, which encodes B-Raf, and one allele of Raf1, which encodes C-Raf, affected DRG neuron maturation as well as proprioceptive axon projection toward the ventral horn in the spinal cord. Finally, conditional elimination of all Braf and Raf1 alleles strongly reduced neurotrophin-dependent axon growth in vitro as well as cutaneous axon terminal arborization in vivo. We conclude that Raf function is crucial for several aspects of DRG neuron development, including differentiation and axon growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Wellbrock, C., Karasarides, M. & Marais, R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol. 5, 875–885 (2004).
Markus, A., Patel, T.D. & Snider, W.D. Neurotrophic factors and axonal growth. Curr. Opin. Neurobiol. 12, 523–531 (2002).
Klinz, F.J., Wolff, P. & Heumann, R. Nerve growth factor-stimulated mitogen-activated protein kinase activity is not necessary for neurite outgrowth of chick dorsal root ganglion sensory and sympathetic neurons. J. Neurosci. Res. 46, 720–726 (1996).
Althini, S., Usoskin, D., Kylberg, A., Kaplan, P.L. & Ebendal, T. Blocked MAP kinase activity selectively enhances neurotrophic growth responses. Mol. Cell. Neurosci. 25, 345–354 (2004).
Markus, A., Zhong, J. & Snider, W.D. Raf and Akt mediate distinct aspects of sensory axon growth. Neuron 35, 65–76 (2002).
Marais, R., Light, Y., Paterson, H.F., Mason, C.S. & Marshall, C.J. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic Ras and tyrosine kinases. J. Biol. Chem. 272, 4378–4383 (1997).
Mercer, K. et al. ERK signalling and oncogene transformation are not impaired in cells lacking A-Raf. Oncogene 21, 347–355 (2002).
Morice, C. et al. Raf-1 and B-Raf proteins have similar regional distributions but differential subcellular localization in adult rat brain. Eur. J. Neurosci. 11, 1995–2006 (1999).
Wojnowski, L., Stancato, L.F., Larner, A.C., Rapp, U.R. & Zimmer, A. Overlapping and specific functions of Braf and Craf-1 proto-oncogenes during mouse embryogenesis. Mech. Dev. 91, 97–104 (2000).
Wiese, S. et al. Specific function of B-Raf in mediating survival of embryonic motoneurons and sensory neurons. Nat. Neurosci. 4, 137–142 (2001).
Mantamadiotis, T. et al. Disruption of CREB function in brain leads to neurodegeneration. Nat. Genet. 31, 47–54 (2002).
Murakami, M.S. & Morrison, D.K. Raf-1 without MEK? Sci. STKE [online] 2001, pe30 (2001).
Barnier, J.V., Papin, C., Eychène, A., Lecoq, O. & Calothy, G. The mouse B-raf gene encodes multiple protein isoforms with tissue-specific expression. J. Biol. Chem. 270, 23381–23389 (1995).
Chen, A.P. et al. Forebrain-specific knockout of B-raf kinase leads to deficits in hippocampal long-term potentiation, learning, and memory. J. Neurosci. Res. 83, 28–38 (2006).
Jesenberger, V. et al. Protective role of Raf-1 in salmonella-induced macrophage apoptosis. J. Exp. Med. 193, 353–364 (2001).
Wojnowski, L. et al. Endothelial apoptosis in Braf-deficient mice. Nat. Genet. 16, 293–297 (1997).
Fariñas, I., Wilkinson, G.A., Backus, C., Reichardt, L.F. & Patapoutian, A. Characterization of neurotrophin and Trk receptor functions in developing sensory ganglia: direct NT-3 activation of TrkB neurons in vivo. Neuron 21, 325–334 (1998).
Kramer, I. et al. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron 49, 379–393 (2006).
Wettschureck, N. et al. Loss of Gq/11 family G proteins in the nervous system causes pituitary somatotroph hypoplasia and dwarfism in mice. Mol. Cell. Biol. 25, 1942–1948 (2005).
Lu, X., Melnick, M.B., Hsu, J.C. & Perrimon, N. Genetic and molecular analyses of mutations involved in Drosophila raf signal transduction. EMBO J. 13, 2592–2599 (1994).
Bennett, D.L. et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J. Neurosci. 18, 3059–3072 (1998).
Zhang, B.-H. & Guan, K.-L. Activation of B-Raf kinase requires phosphorylation of the conserved residues Thr598 and Ser601. EMBO J. 19, 5429–5439 (2000).
Marek, L. et al. Multiple signaling conduits regulate global differentiation-specific gene expression in PC12 cells. J. Cell. Physiol. 201, 459–469 (2004).
Chen, C.L. et al. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron 49, 365–377 (2006).
Chen, A.I., de Nooij, J.C. & Jessell, T.M. Graded activity of transcription factor Runx3 specifies the laminar termination pattern of sensory axons in the developing spinal cord. Neuron 49, 395–408 (2006).
Inoue, K. et al. Runx3 controls the axonal projection of proprioceptive dorsal root ganglion neurons. Nat. Neurosci. 5, 946–954 (2002).
Patel, T.D. et al. Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron 38, 403–416 (2003).
Molliver, D.C. et al. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19, 849–861 (1997).
Patel, T.D., Jackman, A., Rice, F.L., Kucera, J. & Snider, W.D. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron 25, 345–357 (2000).
Kuruvilla, R. et al. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell 118, 243–255 (2004).
Galabova-Kovacs, G. et al. Essential role of B-Raf in ERK activation during extraembryonic development. Proc. Natl. Acad. Sci. USA 103, 1325–1330 (2006).
Rodriguez-Viciana, P. et al. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science (2006).
Niihori, T. et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat. Genet. 38, 294–296 (2006).
Offermanns, S. et al. Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Gαq. Proc. Natl. Acad. Sci. USA 94, 14089–14094 (1997).
Wan, P.T. et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116, 855–867 (2004).
Garnett, M.J., Rana, S., Paterson, H., Barford, D. & Marais, R. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol. Cell 20, 963–969 (2005).
Rushworth, L.K., Hindley, A.D., O'Neill, E. & Kolch, W. Regulation and role of Raf-1/B-Raf heterodimerization. Mol. Cell. Biol. 26, 2262–2272 (2006).
Galabova-Kovacs, G. et al. ERK and beyond: insights from B-Raf and Raf-1 conditional knockouts. Cell Cycle 5, 1514–1518 (2006).
Marmigere, F. et al. The Runx1/AML1 transcription factor selectively regulates development and survival of TrkA nociceptive sensory neurons. Nat. Neurosci. 9, 180–187 (2006).
Ito, Y. Oncogenic potential of the RUNX gene family: 'overview'. Oncogene 23, 4198–4208 (2004).
Pierchala, B.A., Tsui, C.C., Milbrandt, J. & Johnson, E.M. NGF augments the autophosphorylation of Ret via inhibition of ubiquitin-dependent degradation. J. Neurochem. 100, 1169–1176 (2007). [AU: Vol/pp supplied; correct?]
Camarero, G. et al. Cortical migration defects in mice expressing A-RAF from the B-RAF locus. Mol. Cell. Biol. 26, 7103–7115 (2006).
Melnick, M.B., Perkins, L.A., Lee, M., Ambrosio, L. & Perrimon, N. Developmental and molecular characterization of mutations in the Drosophila-raf serine/threonine protein kinase. Development 118, 127–138 (1993).
Hagemann, C. & Rapp, U.R. Isotype-specific functions of Raf kinases. Exp. Cell Res. 253, 34–46 (1999).
Tapinos, N. & Rambukkana, A. Insights into regulation of human Schwann cell proliferation by Erk1/2 via a MEK-independent and p56Lck-dependent pathway from leprosy bacilli. Proc. Natl. Acad. Sci. USA 102, 9188–9193 (2005).
Giroux, S. et al. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 9, 369–376 (1999).
Bélanger, L.-F. et al. Mek2 Is dispensable for mouse growth and development. Mol. Cell. Biol. 23, 4778–4787 (2003).
Hatano, N. et al. Essential role for ERK2 mitogen-activated protein kinase in placental development. Genes Cells 8, 847–856 (2003).
Nekrasova, T. et al. ERK1-deficient mice show normal T cell effector function and are highly susceptible to experimental autoimmune encephalomyelitis. J. Immunol. 175, 2374–2380 (2005).
Yao, Y. et al. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc. Natl. Acad. Sci. USA 100, 12759–12764 (2003).
Acknowledgements
The authors wish to thank L. Lei, L. Parada (University of Texas Southwestern), L. Reichardt (University of California San Francisco), T. Jessell (Columbia University) and E. Turner (University of California San Diego) for sharing valuable antibodies, P. Barker (McGill University) for the PC12 cells and K.L. Guan (University of Michigan, Ann Arbor) for the BRAF-ED cDNA. We are grateful to P. Ye and A.J. D'Ercole for blood glucose assays and pituitary gland study. Special thanks are due to the Animal Model Core Facility at University of North Carolina at Chapel Hill for their expert assistance in generating the gene-targeted mice. This work was supported by RO1N0S31768 from the US National Institutes of Health and the Confocal and Multiphoton Microscopy Core Facility supported by National Institute of Neurological Disorders and Stroke center grant NS45892.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Schematic showing the Braf exon-3 gene targeting construct with chromosomal location of the loxP sites. (PDF 261 kb)
Supplementary Fig. 2
Loss of DRG neuron markers in E12.5 Braf−/− embryos. (PDF 522 kb)
Supplementary Fig. 3
Homeostasis abnormalities in Braff/−nesCre+ mice. (PDF 246 kb)
Supplementary Fig. 4
At P30, the brain size of Braff/−nesCre+ mice is reduced, compared with control littermates'. (PDF 2494 kb)
Supplementary Fig. 5
Profiling of transcription factor activities induced by NGF and B-Raf in PC12 cells. (PDF 4832 kb)
Supplementary Fig. 6
Phenotype in Braff/fRaf1f/+nesCre+ mice. (PDF 3549 kb)
Supplementary Fig. 7
Loss of B- and C-Raf at early embryonic stages impairs NGF-induced axon growth but not neuron survival. (PDF 491 kb)
Supplementary Table 1
List of selected transcription factor complexes activated by NGF or B-Raf 24 h after stimulation. (PDF 136 kb)
Supplementary Video 1
A Braff/−nesCre+ mouse and its Braff/− littermate at P30. The B-Raf conditional null mice are easy to recognize as they are much smaller than their control littermates. Note that mice appear to be hyperactive and clearly have full locomotor activity. This latter observation demonstrates that motor and sensory neurons have survived into adulthood. (MPG 4152 kb)
Supplementary Video 2
A Raf1f/−nesCre+ mouse and its control littermate at the age of P30. The C-Raf conditional nulls have no obvious behavioral phenotype. (MPG 665 kb)
Rights and permissions
About this article
Cite this article
Zhong, J., Li, X., McNamee, C. et al. Raf kinase signaling functions in sensory neuron differentiation and axon growth in vivo. Nat Neurosci 10, 598–607 (2007). https://doi.org/10.1038/nn1898
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1898
This article is cited by
-
Know Your Model: A knockout does not always make a null
Lab Animal (2020)
-
Leucine-485 deletion variant of BRAF may exhibit the severe end of the clinical spectrum of CFC syndrome
Journal of Human Genetics (2019)
-
iTRAQ quantitatively proteomic analysis of the hippocampus in a rat model of accumulative microwave-induced cognitive impairment
Environmental Science and Pollution Research (2019)
-
Patient-derived iPSCs show premature neural differentiation and neuron type-specific phenotypes relevant to neurodevelopment
Molecular Psychiatry (2018)
-
Cellular model of neuronal atrophy induced by DYNC1I1 deficiency reveals protective roles of RAS-RAF-MEK signaling
Protein & Cell (2016)