Abstract
Pre-determining fetal sex is against the random and equal opportunity that both conceptus sexes have by nature. Yet, under a wide variety of circumstances, populations shift their birth sex ratio from the expected unity. Here we show, using fluorescence in situ hybridization, that in a population of pygmy hippopotamus (Choeropsis liberiensis) with 42.5% male offspring, males bias the ratio of X- and Y-chromosome-bearing spermatozoa in their ejaculates, resulting in a 0.4337±0.0094 (mean±s.d.) proportion of Y-chromosome-bearing spermatozoa. Three alternative hypotheses for the shifted population sex ratio were compared: female counteract male, female indifferent, or male and female in agreement. We conclude that there appears little or no antagonistic sexual conflict, unexpected by prevailing theories. Our results indicate that males possess a mechanism to adjust the ratio of X- and Y-chromosome-bearing spermatozoa in the ejaculate, thereby substantially expanding currently known male options in sexual conflict.
Similar content being viewed by others
Introduction
Both male and female gametes are produced through the process of meiosis. It is usually assumed that because an equal number of X- and Y-chromosome-carrying spermatozoa are produced during spermatogenesis1, any observed shift from a 1:1 sex ratio among offspring must be the result either of differences in the fertilizing ability of the two gamete types2,3 or cryptic female choice4. Factors such as population density5, female social status6, female body condition or resource availability7 and stress8 are known to 'shift' sex ratios away from the expected equal birth sex ratio, but the biological mechanism through which a mammal (humans included) can and will bias offspring sex ratio is still unknown.
Noting the difference between the sexes in terms of investment in offspring and the benefits gained thereby, it has been assumed that owing to its usually much larger investment, the female has considerably more to win or lose, and if mechanisms to bias offspring sex ratios do exist, they would be operated by the female9. Hence, both empirical and theoretical work has traditionally concentrated on the female and dismissed any potential paternal contribution as minor and irrelevant.
Still, it is surprising how small is the number of studies that have evaluated the ratio between the X- and Y-chromosome-bearing spermatozoa, the so-called 'primary sex ratio'. Most available studies, conducted on samples from boar10, cattle10,11,12,13,14 or humans15,16,17, used inaccurate evaluation techniques such as fluorescence staining of the F-body or PCR amplification on pooled semen samples. Correlation between the ratio in the ejaculate and the birth sex ratio was found in those studies that made the comparison12,16,17. The few studies that were based on the more accurate fluorescence in situ hybridization (FISH) evaluation technique18,19,20 either suffered from a considerably smaller sample size compared with their control (176 samples compared with the pooled national data on birth sex ratio from several European countries and the United States) and counted too few cells to detect a sex ratio variation (only 200 cells per ejaculate)18, dealt with samples obtained from infertile patients (where actually an excess of X-chromosome-bearing spermatozoa was detected)19, or had too small sample size to detect a small difference (N=7 compared with N=12)20.
As offspring sex is the result of the combined inputs of both parents and their interaction, three scenarios of sexual conflict can be envisaged: antagonistic interests, one sex neutral or coinciding interests. Under the first scenario, if it is in the male interest to bias sex ratio in favour of females, the female interest might be to bias the sex ratio in favour of male offspring, for instance by activating mechanisms that increase fertilization by Y-chromosome-bearing spermatozoa or by selective death of female embryos. If selection intensity is equal or in favour of the females, male and female inputs would be likely to cancel each other out and result in a sex ratio at birth of ∼0.5, or higher. In the second scenario, there is either a lower selection pressure on the females to counteract male interests or the females' interests are neutral with respect to the male interest. In this case, the female input will be ∼0.5. According to the third scenario, male and female interests coincide and there is effectively no sexual conflict. In this case, both male and female input should be shifted in the same direction.
The endangered pygmy hippopotamus (Choeropsis liberiensis), an endemic species to waterways and forests of West Africa in Sierra Leone, Guinea, Ivory Coast and Liberia, resembles the common hippopotamus but is much smaller in size. It is a long-lived, solitary species where the sexes only meet for mating and where males defend territories, sometimes in fierce fights with competing males, which may have fatal consequences21. While little is known about their reproduction in the wild, the captive population reproduces well. In the captive population, offspring sex ratio is shifted towards an excess of females—at birth only 41% of offspring were reported to be males22. The cause of this shift and the mechanism by which it is achieved are unknown.
While most sex ratio skews investigated to date were believed to be due to the female, this study was designed to challenge this assumption by showing that male-based offspring sex ratio-shifting mechanism may also exist. We therefore investigated semen samples obtained from pygmy hippopotamus males and found the ratio of X- and Y-chromosome-bearing spermatozoa in these samples to be similar to the sex ratio at birth, as derived from an examination of the most recent data on the captive pygmy hippopotamus population23. Using both sources of information, we derived the likely influence of any female post-copulatory mechanism to assess the direction and strength of male and female biases in terms of sexual conflict. Our analysis suggests the existence of little or no antagonistic sexual conflict in the study population where males seem to possess a mechanism to actively shift the sex ratio in their ejaculates.
Results
Fluorescence in situ hybridization
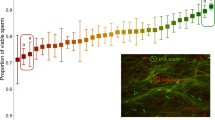
After applying FISH, spermatozoa from all individuals were successfully painted with the X- and Y-chromosome-specific dyes. Accordingly, the mean sex ratio of all samples was biased in favour of X-chromosome-bearing spermatozoa, as the mean proportion of Y-chromosome-bearing spermatozoa was 0.4337 (s.d.=0.0094, Wilcoxon signed-ranks test, P=0.005, N=10, Table 1). The sex ratio of the sampled sperm was skewed towards an excess of X-chromosome-bearing spermatozoa in all individuals and significantly differed from the expected ratio of 0.5 (binomial test, P<0.00001 in all cases, Table 1). Plotting ejaculated sperm sex ratio against bull age showed no correlation (Spearman correlation coefficient=−0.152, P=0.674).
FISH efficiency, evaluated as the ratio of stained cells to the total number of cells counted, was 97.61±1.65% (mean±s.d.; N=10). The proportion of ambiguous staining was 1.43±0.85% (mean±s.d.; N=10).
Studbook analysis
The 2008 pygmy hippopotamus studbook lists 1,089 captive births between 1919 and 2008. Of these, 32 births were of infants with unknown sex. The sex ratio of all captive births of known sex was 0.4248 (449 males out of 1057 births), significantly lower than the expected unity (binomial test, P<0.0001) but similar to the observed ratio between X- and Y-chromosome-bearing spermatozoa observed in this study (binomial test, one-tailed, P=0.290). The sex ratio of animals that survived to the age of 5 years was 0.3829 (242 males out of 632 captive born pygmy hippopotami surviving to adulthood), significantly lower than the expected 0.5 ratio (binomial test, P<0.0001). Thirty nine percent (425/1,089) of all captive born individuals died before the age of 5 years. The studbook lists 72 wild-born males and 85 wild-born females, a ratio of 0.4586, not different from unity (binomial test, P=0.338). There are 130 living males listed in the studbook, 103 of them are adults being over five years old.
The current captive pygmy hippopotamus population has 61 founders, 44.39 of their genomes still survive. The average inbreeding coefficient of the population is 0.0666, with gene diversity of 0.9699 and mean kinship coefficient of 0.0301. Mean kinship coefficient of the individual males in this study ranged between 0.012 and 0.053 (Table 2). There was no correlation between individual mean kinship coefficient and its sperm sex ratio (Spearman correlation coefficient=−0.322, P=0.361, N=9).
Of the ten males participating in this study, three never sired offspring (Table 2). Comparison of offspring sex ratio of the other bulls to the expected 0.5 found no differences in any of them (binomial test, P>0.05 for all).
Evaluation of sexual conflict scenarios
Evaluations of the three possible scenarios were made with reference to the observed population sex ratio at birth: antagonistic interests, one sex neutral and coinciding interests.
For antagonistic interests, if selection intensity is equal or in favour of females, the observed male input of 0.4337 (95% confidence interval (CI): 0.4270–0.4404) and female input would be likely to cancel each other out and result in a sex ratio at birth of ∼0.5, or higher. This would require a female bias in the order of 0.5663 (95% CI: 0.5366–0.5963) or higher. The observed sex ratio at birth of 0.4258 is significantly below that.
For one sex neutral, the female input will be ∼0.5 (95% CI: 0.4704–0.5306). In combination with the male input of 0.4337 (95% CI: 0.4270–0.4404), the resulting sex ratio would be in the range of 0.4487–0.4855, approaching or significantly higher than the observed sex ratio (binomial test, one-tailed, P=0.063 for lower value and P=0.00004 for higher value).
For coinciding interests, the male and female inputs are shifted in the same direction. Given the observed male input of 0.4337 (95% CI: 0.4270–0.4404), the required female bias to produce the observed population sex ratio (0.4248) is 0.4159 (95% CI: 0.3869–0.4462).
Discussion
The results indicate that male and female interests most probably converged and that both male (0.4337) and female (0.4159) biases in sex allocation result in the production of an excess of female offspring. Although remote (P=0.063 for lower CI value), the possibility that the female is neutral to the outcome and the bias is by the males only cannot be ruled out. It is also possible that the females cannot counter the males' skew because the selective pressure on the captive population did not act long enough to produce a response. Owing to the small number of offspring produced by each male, no shifts from the expected 0.5 sex ratio were or could be detected.
A comparison of these skewed sex ratios in the captive population with data from free-ranging populations is currently not possible, as there are no data from wild populations. Such data will be difficult to obtain because male and female pygmy hippopotamus have no external distinctive features. The next best comparison is a comparison of sex ratios of wild-born male and female pygmy hippopotami in the international studbook. The observed ratio of 0.4586 among wild-caught animals in the population did not significantly differ from the expected 0.5. Hidden selection bias may exist in the probability of catching males or females, as is the case for instance in cheetahs25, or in post-capture selection.
In the study population, the already skewed secondary sex ratio was further biased in favour of females at adulthood because male infants and juveniles were more likely to die than their female counterparts. This results in an even stronger female-biased sex ratio at adulthood of 0.3829 and a significantly lower sex ratio than the ratio of wild-born pygmy hippopotami in the captive population (binomial test, one-tailed, P<0.0001).
Why should males opt to produce more females? If a male is long-lived, the ownership of a territory being a pre-condition to mating success, and the habitat is saturated with occupied territories, then sons are unlikely to find an empty territory and are likely to compete with their fathers for territory ownership. Under such conditions, fathers have an interest in directing the sex of the progeny in a direction that will enable it to increase its own reproductive success and avoid father–son competition.
How could males decide on producing more females? In order to alter the ratio of X- and Y-chromosome-bearing spermatozoa in their ejaculate, male pygmy hippopotami would benefit from a mechanism activated by environmentally induced stimuli. One possible stimulus previously shown to induce a sex ratio bias in favour of females is high population density5. There is currently no information on densities of free-ranging pygmy hippopotamus populations in their natural habitat. They are known to live mostly a solitary life in and around the streams and swamps of the densely forested regions of West Africa26, with the two sexes joining only for mating. At other times, male–male or male–female encounters often take an aggressive course and fighting may lead to severe injuries and death21. When in captivity, animals are usually housed in the same enclosure. Even when males and females are separated from each other, they would still have visual, olfactory or auditory contact with nearby animals. The captive enclosure and proximity to other pygmy hippopotami may convey a sense of high population density, thereby possibly activating mechanisms that could induce a shift in sex ratio. Female-biased sex allocation by both parents suggests that females are the dispersing sex in pygmy hippopotamus. Thus, by producing more females, parents could reduce competition over local resources7.
Other possible causes can also be envisioned. Scrotal heat stress was shown to produce a bias in the resulting foetus sex ratio in mice if mating took place on the day of heat stress, though it had no effect on epididymal sperm sex ratio27. In our study, scrotal heat stress can be ruled out for several reasons: in hippopotami there is no scrotum and the testes descend only as far as the inguinal canal so they are less affected by environmental temperature changes. All males evaluated for this study reside in zoos in the temperate zone, and heat stress was not an issue on or around any of the collection days. Furthermore, pygmy hippopotamus enclosures in all zoos contain a wallowing pool where animals can immerse to cool themselves when needed. Finally, whatever effect heat stress might have, it is not on the ratio between X- and Y-chromosome-bearing spermatozoa but rather on survival of the embryos/foetuses. Another possible mechanism is the correlation reported in humans between paternal age and offspring sex ratio28,29. Evaluation of the sampled sperm sex ratio against bulls' age in the present study found no such correlation. Inbreeding might be yet another possibility. Inbreeding is known to cause a decline in sperm quality30 and thus might also affect its sex ratio. The captive pygmy hippopotamus, however, cannot be considered highly inbred having an average inbreeding coefficient of 0.0666 and gene diversity of 0.969931. Evaluation of the mean kinship coefficient of the males in this study against their sperm sex ratio found no such correlation. Coital rate was also reported to affect offspring sex ratio32,33. High coital rate, however, is not an issue in pygmy hippopotami as males and females are often housed separately to avoid between-individual aggression. Finally, the issue of stress should also be considered. It was shown, for instance in humans, that offspring sex ratio declines when the parents are subjected to chronic34 or acute8 stress. Causes for such stress, including the perception of high population density mentioned above or other issues related to captivity, might exist in the study population though it has never been evaluated. Regardless of what the cause might be, and even if the reasons for the bias in male and female sex allocation may not be the same in this population, the outcome is in agreement.
Spermatozoa carrying X-chromosome or Y-chromosome show very small or no phenotypic differences35. Regardless of whether the observed shift in ejaculated and epididymal sperm sex ratio has an adaptive or non-adaptive explanation, our data suggest the existence of a mechanism that selectively favours or eliminates spermatozoa of a specific type. What this mechanism might be can only be speculated on at this stage. Possible mechanisms that can be considered include selective removal during spermatogenesis, meiotic drive, selective apoptosis or selective epididymal sperm phagocytosis. Although a single male cannot provide any concrete answer, the fact that the ratio found in epididymal sperm was similar to that found in ejaculated sperm suggests that when looking for such a mechanism, we should concentrate on the testes and whatever takes place in them. If this mechanism is not unique to the pygmy hippopotamus but an integral part of male mammalian reproduction at large, it may provide an alternative explanation for a number of sex ratio shifts, such as the excess in human male births36, which hitherto have been explained by female cryptic choice.
Methods
Animals
Sperm was collected from captive pygmy hippopotami by electroejaculation as part of a general assessment of reproductive health (N=9) or from the cauda epididymis following euthanasia (N=1). These ten males constitute 10% of the entire world captive adult male population in this species. Semen assessment results, reported elsewhere24 (and Table 1 therein), demonstrated that the ejaculates were of good to excellent quality. Ages of the males ranged between 9 and 40 years.
Materials
Unless otherwise mentioned, all materials were of reagent grade or higher, and were purchased from Sigma–Aldrich Chemie GmbH, Taufkirchen, Germany.
Anaesthesia
All semen electroejaculation collection procedures were conducted under general anaesthesia. Animals were injected in the neck muscles by remote darts with a combination of medetomidine (Zalopine®, Orion Farmos Corporation, Espoo, Finland) at a dosage of 0.08 mg kg−1 and ketamine (WDT eG, Garbsen, Germany) at 1.2 mg kg−1 on the basis of estimated body mass. Animals were then manually intubated using a long-blade laryngoscope and maintained with isoflurane (Isobo®, Nbl. Der Essex Pharma GmbH, Munich, Germany) and air, air mixed with oxygen or pure oxygen throughout the procedure. Anaesthesia was partially antagonized by intramuscular injection of atipamezole hydrochloride (Antisedan, Pfizer GmbH, Karlsruhe, Germany) at five times the dosage of medetomidine. All animals had sufficiently recuperated to be up and standing within 10 to 15 min after application of the antidote.
Semen collection and evaluation
Semen from nine of the males was collected by electroejaculation and assessed in terms of volume, concentration, morphology, acrosome integrity and subjective total motility, as previously described24 and Table 1 therein. Seminal plasma osmolarity (N=4) and resistance to hypoosmotic conditions (N=3) were also evaluated (ref. 24 and Table 1 therein). Cauda epididymal spermatozoa sample (N=1) was obtained by removal of the testes from the inguinal canal following euthanasia. The tunicas were excised and the epididymis was removed. Cuts were made in the cauda epididymis with sterile surgical scalpel, and the spermatozoa were aspirated and transferred directly into Carnoy's fixative for preparation (see next section).
Semen preparation and FISH
Semen was washed at least three times in freshly made, ice-cold Carnoy's fixative (glacial acetic acid and methanol at a ratio of 1:3). Each washing step included suspending the sample in 8 ml fixative, vortexing and then centrifugation at 400 g for 10 min. After centrifugation the supernatant was discarded. Subsequent to the final washing, the semen sample was resuspended in ice-cold fixative and kept at −20 °C pending further treatment.
For the FISH procedure, the fixed semen sample was vortexed and one drop of the suspension was placed on a microscopic slide and set aside horizontally to air dry. The position of the drop was marked with a glasscutter. A 500 μl of 10 mM dithioerythritol in 0.1 M Tris-aminomethan (Tris base) were applied to each slide, covered with parafilm and incubated at room temperature for 15 min. Following incubation, the parafilm was removed, excess liquid discarded and the slides were lightly washed in 2×SSC (saline–sodium citrate) buffer solution at room temperature. After washing, the slides were left to air dry and then 100 μl of 70% (v/v) formamid (Carl Roth GmbH+Co., Karlsruhe, Germany)/30% (v/v) 2×SSC were applied to each slide, covered with a cover slip and incubated for 10 min at 80 °C in a slide processing system (ThermoBrite, Abbott, Wiesbaden, Germany). At the end of incubation, the cover slip was removed and slides were washed in cold distilled water and then left to air dry. Subsequently, slides were dehydrated in an ice-cold ethanol series (70%, 90%, 100%, 1 min in each) and set aside to air dry thoroughly. When ready, 1 μl of each of the pygmy hippopotamus X- and Y-chromosome-specific FISH probes (Cambridge Resource Centre for Comparative Genomics, Cambridge, UK) was mixed with 8 μl of hybridization mix, applied to the marked area on the slide, covered with a cover slip and sealed with rubber cement. The X-chromosome probe was labelled with Cy3 (fluorescence red) and the Y-chromosome probe was labelled with FITC (fluorescence green). The probe and target DNA were co-denatured using the ThermoBrite system set at 65 °C for 10 min followed by hybridization at 37 °C overnight. After hybridization, rubber cement and cover slip were removed, and slides were washed for 1 min in 0.4×SSC at 75 °C followed by 1 min in 2×SSC/0.1% (v/v) NP-40 at room temperature. After air drying in the dark, slides were counterstained with Vectashield antifade solution with 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA, USA) and covered with a cover slip. Slides were assessed under a fluorescence microscope (BZ-8000; Keyence Corporation, Osaka, Japan) equipped with an ×60 oil immersion lens. At least 2,000 cells were assessed per ejaculate. Cells with ambiguous staining were excluded from analysis.
Studbook analysis
The most recent international pygmy hippopotamus studbook available at the time of the study23, updated till 31 December 2008, was analysed. Data extracted from the studbook on all captive births included sex, sire, dam, dam's origin (captive or wild-born), birth date, birth location and death date. Animals that died before the age of 5 were regarded as dying prematurely. Calves with unknown sex were excluded from sex ratio analysis. Data on the genetic diversity of the population and the mean kinship coefficient as well as information about offspring of the males participating in this study were also extracted from the studbook.
Statistical analysis
Throughout, the sex ratio is expressed as the fraction of males or Y-chromosome-bearing spermatozoa in a sample or population. All binomial tests were exact tests based on cumulative probabilities and, unless otherwise stated, all tests (Spearman correlation coefficient, binomial and Wilcoxon signed-ranks test) are two-tailed. Statistical tests were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA) and PASW Statistics 18.0.0 (IBM Corporation, NY, USA) statistical software. Differences were considered significant when P<0.05. The modified Wald method37 was used to calculate CIs.
Additional information
How to cite this article: Saragusty, J. et al. Male pygmy hippopotamus influence offspring sex ratio. Nat. Commun. 3:697 doi: 10.1038/ncomms1700 (2012).
References
McClung, C. E. The accessory chromosome - sex determinant? Biol. Bull. 3, 43–84 (1902).
Shettles, L. B. After office hours conception and birth sex ratios: a review. Obstet. Gynecol. 18, 122–130 (1961).
Martin, J. F. Changing sex ratios: the history of Havasupai fertility and its implications for human sex ratio variation. Curr. Anthropol. 35, 255–280 (1994).
Lindahl, P. E. & Sundell, G. Sex ratio in the golden hamster before uterine implantation. Nature 182, 1392 (1958).
Kruuk, L. E., Clutton-Brock, T. H., Albon, S. D., Pemberton, J. M. & Guinness, F. E. Population density affects sex ratio variation in red deer. Nature 399, 459–461 (1999).
Simpson, M. J. A. & Simpson, A. E. Birth sex ratios and social rank in rhesus monkey mothers. Nature 300, 440–441 (1982).
Clark, A. B. Sex ratio and local resource competition in a prosimian primate. Science 201, 163–165 (1978).
Zorn, B., Sucur, V., Stare, J. & Meden-Vrtovec, H. Decline in sex ratio at birth after 10-day war in Slovenia: brief communication. Hum. Reprod. 17, 3173–3177 (2002).
Grant, V. J. & Martin, J. F. On sex ratio and coital rate: a hypothesis without foundation. Curr. Anthropol. 36, 295–298 (1995).
Chandler, J. E., Steinholt-Chenevert, H. C., Adkinson, R. W. & Moser, E. B. Sex ratio variation between ejaculates within sire evaluated by polymerase chain reaction, calving, and farrowing records. J. Dairy Sci. 81, 1855–1867 (1998).
Chandler, J. E., Canal, A. M., Paul, J. B. & Moser, E. B. Collection frequency affects percent Y-chromosome bearing sperm, sperm head area and quality of bovine ejaculates. Theriogenology 57, 1327–1346 (2002).
Chandler, J. E. et al. Calving sex ratio as related to the predicted Y-chromosome-bearing spermatozoa ratio in bull ejaculates. Theriogenology 67, 563–571 (2007).
Madrid-Bury, N. et al. Effect of ejaculate, bull, and a double swim-up sperm processing method on sperm sex ratio. Zygote 11, 229–235 (2003).
Szyda, J., Simianer, H. & Lien, S. Sex ratio distortion in bovine sperm correlates to recombination in the pseudoautosomal region. Genet. Res. 75, 53–59 (2000).
Lobel, S. M., Pomponio, R. J. & Mutter, G. L. The sex ratio of normal and manipulated human sperm quantitated by the polymerase chain reaction. Fertil. Steril. 59, 387–392 (1993).
Dmowski, W. P., Gaynor, L., Rao, R., Lawrence, M. & Scommegna, A. Use of albumin gradients for X and Y sperm separation and clinical experience with male sex preselection. Fertil. Steril. 31, 52–57 (1979).
Bibbins, P. E. Jr., Lipshultz, L. I., Ward, J. B. Jr. & Legator, M. S. Fluorescent body distribution in spermatozoa in the male with exclusively female offspring. Fertil. Steril. 49, 670–675 (1988).
Graffelman, J., Fugger, E. F., Keyvanfar, K. & Schulman, J. D. Human live birth and sperm-sex ratios compared. Hum. Reprod. 14, 2917–2920 (1999).
Johannisson, R. et al. Increased frequency of X-bearing sperm in males from an infertility clinic: analysis by two-color fluorescence in situ hybridization. Cytogenet. Genome Res. 98, 240–244 (2002).
Irving, J., Bittles, A., Peverall, J., Murch, A. & Matson, P. The ratio of X- and Y-bearing sperm in ejaculates of men with three or more children of the same sex. J. Assist. Reprod. Genet. 16, 492–494 (1999).
Rahn, P. On housing the pygmy hippopotamus. Int. Zoo Yb. 18, 187–190 (1978).
Zschokke, S. Distorted sex ratio at birth in the captive pygmy hippopotamus, Hexaprotodon liberiensis. J. Mammal. 83, 674–681 (2002).
Steck, B. & Pagan, O. Pygmy Hippopotamus Choeropsis liberiensis (Morton, 1844) International Studbook 2008 15th edn (Basel Zoo, 2008).
Saragusty, J., Hildebrandt, T. B., Bouts, T., Göritz, F. & Hermes, R. Collection and preservation of pygmy hippopotamus (Choeropsis liberiensis) semen. Theriogenology 74, 652–657 (2010).
Marker, L. L., Dickman, A. J., Mills, M. G. L. & Macdonald, D. W. Aspects of the management of cheetahs, Acinonyx jubatus jubatus, trapped on Namibian farmlands. Biol. Conserv. 114, 401–412 (2003).
Schomburgk, H. On the trail of the pygmy hippo, an account of the Hagenbeck expedition to Liberia. Zool. Soc. Bull. 16, 880–884 (1912).
Pérez-Crespo, M., Pintado, B. & Gutiérrez-Adán, A. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol. Reprod. Dev. 75, 40–47 (2008).
Ruder, A. Paternal-age and birth-order effect on the human secondary sex ratio. Am. J. Hum. Genet. 37, 362–372 (1985).
Novitski, E. & Sandler, L. The relationship between parental age, birth order and the secondary sex ratio in humans. Ann. Hum. Genet. 21, 123–131 (1956).
Ruiz-Lopez, M. J., Evenson, D. P., Espeso, G., Gomendio, M. & Roldan, E. R. High levels of DNA fragmentation in spermatozoa are associated with inbreeding and poor sperm quality in endangered ungulates. Biol. Reprod. 83, 332–338 (2010).
Steck, B. & Pagan, O. Pygmy Hippopotamus Choeropsis liberiensis (Morton, 1844) International Studbook 2009 16th edn (Basel Zoo, 2010).
Hays, F. A. The influence of excessive sexual activity of male rabbits. II. On the nature of their offspring. J. Exp. Zool. 25, 571–613 (1918).
Bartoš, L. & Trojan, S. Effect of prolonged frequency of mating by the male rat on his fertility and on the sex ratio of his offspring. Anim. Reprod. Sci. 17, 271–279 (1988).
Catalano, R. A. Sex ratios in the two Germanies: a test of the economic stress hypothesis. Hum. Reprod. 18, 1972–1975 (2003).
Seidel, G. E. Jr. Sexing mammalian spermatozoa and embryos - state of the art. J. Reprod. Fertil. Suppl. 54, 477–487 (1999).
The World Factbook. 〈https://www.cia.gov/library/publications/the-world-factbook/index.html〉 (2009).
Agresti, A. & Coull, B. A. Approximate is better than 'exact' for interval estimation of binomial proportions. Am. Stat. 52, 119–126 (1998).
Acknowledgements
We wish to thank Ms Beatrice Steck, International studbook keeper of pygmy hippopotamus, for her support to this project, and Ms Nana Satake from the Taronga Western Plains Zoo, Australia, for her help in sample collection.
Author information
Authors and Affiliations
Contributions
J.S., R.H, F.G. and T.B.H. designed the research, J.S., R.H, T.B., F.G. and T.B.H conducted the research, J.S. and H.H. analysed the data, J.S. and H.H. wrote the paper and R.H., T.B., F.G. and T.B.H. edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Saragusty, J., Hermes, R., Hofer, H. et al. Male pygmy hippopotamus influence offspring sex ratio. Nat Commun 3, 697 (2012). https://doi.org/10.1038/ncomms1700
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms1700
This article is cited by
-
Detection of unamplified target genes via CRISPR–Cas9 immobilized on a graphene field-effect transistor
Nature Biomedical Engineering (2019)
-
Hippo sperm discriminates against the male sex
Nature Middle East (2012)
-
الحيوانات المنوية لفرس النهر تميز ضد جنس الذكور
Nature Middle East (2012)
-
Should he stay or should he go: male influence on offspring sex ratio via postcopulatory attendance
Behavioral Ecology and Sociobiology (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.