Abstract
A double-negative feedback loop formed by the morning genes CIRCADIAN CLOCK ASSOCIATED1 (CCA1)/LATE ELONGATED HYPOCOTYL (LHY) and the evening gene TIMING OF CAB EXPRESSION1 (TOC1) contributes to regulation of the circadian clock in Arabidopsis. A 24-h circadian cycle starts with the peak expression of CCA1 at dawn. Although CCA1 is targeted by multiple transcriptional repressors, including PSEUDO-RESPONSE REGULATOR9 (PRR9), PRR7, PRR5 and CCA1 HIKING EXPEDITION (CHE), activators of CCA1 remain elusive. Here we use mathematical modelling to infer a co-activator role for LIGHT-REGULATED WD1 (LWD1) in CCA1 expression. We show that the TEOSINTE BRANCHED 1-CYCLOIDEA-PCF20 (TCP20) and TCP22 proteins act as LWD-interacting transcriptional activators. The concomitant binding of LWD1 and TCP20/TCP22 to the TCP-binding site in the CCA1 promoter activates CCA1. Our study reveals activators of the morning gene CCA1 and provides an action mechanism that ensures elevated expression of CCA1 at dawn to sustain a robust clock.
Similar content being viewed by others
Introduction
The operation of the circadian clock allows organisms to synchronize the internal biological activities with the external 24-h rhythmic light/dark or temperature cues. Plants with a defective circadian clock have reduced fitness1. The circadian clock in Arabidopsis is mainly driven by a double-negative feedback loop formed by the morning-phased CIRCADIAN CLOCK ASSOCIATED1 (CCA1)/LATE ELONGATED HYPOCOTYL (LHY) and the evening-phased TIMING OF CAB EXPRESSION1 (TOC1) (refs 2, 3, 4, 5). In the current model of the Arabidopsis circadian clock, the key driving force for the daily oscillation is based on the repressilatory machinery that uses a repressor of a repressor as an activator to increase the expression of clock genes6,7. In this model, CCA1 is a transcriptional repressor functionally overlapping with LHY for suppressing TOC1 in the morning2. Reciprocally, TOC1 binds to the CCA1 promoter to suppress CCA1 expression in the evening8,9. In addition to TOC1, multiple repressors including PSEUDO-RESPONSE REGULATOR9 (PRR9), PRR7, PRR5 and CCA1 HIKING EXPEDITION (CHE) suppress CCA1 at different times of the day10,11,12.
Although a repressilator-based system can oscillate, the robustness could be enhanced by the integration of a positive feedback loop13. One such positive-feedback loop in the Arabidopsis circadian clock is formed by LIGHT-REGULATED WD1 (LWD1) and PRR9 (ref. 14). More recently, an activator and co-activator system consisting of REVEILLE8 (RVE8) and NIGHT LIGHT-INDUCIBLE AND CLOCK-REGULATEDs (LNKs) could directly target the promoters of evening genes, PRR5 and TOC1, to activate their expression15. However, although the light-activated expression of CCA1 prompts the synchronization of an oscillating clock, activators for CCA1 expression at dawn are still unknown.
Here, our mathematical models show that the simultaneous activation of PRR9 and CCA1 by LWD1 in the clock system allows for more precise predictions of clock behaviours in both wild-type plants and clock mutants. We further demonstrated that by direct interaction with two transcription factors, TEOSINTE BRANCHED 1-CYCLOIDEA-PCF20 (TCP20) and TCP22, LWD1 could associate with the CCA1 promoter in vivo. TCP20 and TCP22 are new clock proteins capable of binding to the TCP-binding site (TBS) in the CCA1 promoter. Without LWDs, the binding of TCP20 and TCP22 to CCA1 promoter fails to activate CCA1. This study first discloses an activator–coactivator complex triggering CCA1 expression in the morning.
Results
The dual activation of PRR9 and CCA1 by LWD1
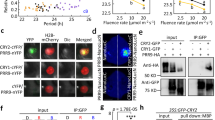
LWD1 can activate PRR9 (ref. 14), one of the transcriptional repressors of CCA1 (ref. 10). However, in contrast to the increased CCA1 level one would expect in the lwd1 lwd2 double mutant with reduced PRR9 expression, CCA1 expression is also much reduced in lwd1 lwd2 (ref. 14). We previously showed that LWD1 can directly target promoters of multiple clock genes, including a weak association with CCA1 at ZT0 (ref. 14). This finding led us to hypothesize that, in addition to activating PRR9, LWD1 can also activate CCA1. To determine the plausibility of such interaction, we used mathematical models to examine clock performance/robustness when LWD1 activates only PRR9 (Model I) or both PRR9 and CCA1 (Model II) (Fig. 1a). Among 2.4 × 108 random parameter sets examined, 1,004 met our criteria of producing oscillation in both the wild type and lwd1 lwd2 mutant (Supplementary Note 1) for Model II as compared with only 27 of 3 × 108 random parameter sets for Model I. Thus, despite having two more parameters to search for in Model II (Supplementary Note 1), the chance of obtaining parameter sets that met our criteria was significantly greater when LWD1 activates both PRR9 and CCA1. Also, only in the circadian system with LWD1 activating both PRR9 and CCA1, but not just PRR9, could the simulation reveal short period lengths of cca1 and toc1 mutants and the long period length of prr9 mutant (Fig. 1a). Including the activation of CCA1 by LWD1 increases the plausibility and robustness of the clock oscillation.
(a) Proposed models for the role of LWD1 in activating the Arabidopsis clock system via PRR9 (Model I) or both PRR9 and CCA1 (Model II). The box plots show period lengths simulated under the representative genetic perturbations (prr9, cca1 or toc1) in these two models, with the wild-type model period being 24 h. The period lengths were calculated from a good majority of the parameter sets obtained (See Supplementary Note 1 for details). Horizontal lines are medians, box edges are interquartile range and whiskers are minimum and maximum. (b) LWD1 associates with the CCA1 promoter in vivo. Diagram shows the gene structure of CCA1. Transcriptional start and exons are marked with arrow and boxes. Black boxes illustrate the coding region. Amplicons ‘a’ and ‘b’ for ChIP-qPCR assays are marked by horizontal black bars. ChIP assays involved use of the anti-LWD1 antibody at ZT21. UBC21 was a negative control. Data are mean±s.d. (n=3 technical replicates). Asterisk indicates that LWD1 preferentially binds with the amplicon ‘b’ in the wild type (Student’s t test; *P<0.001). Similar results were observed in three independent experiments.
To account for the possible limitations inherent to these simplified models, we also assessed the impact of LWD1 on PRR9 alone or both PRR9 and CCA1 by integrating the regulation into a more comprehensive and established model. We chose the model by Pokhilko et al.16, involving many known clock genes, which can replicate the multiple genetic perturbations observed in the experimental results. This model has also been extended to simulate and interpret the input17 and output pathways18. The sole activation of PRR9 by LWD1 in the implemented model failed to simulate the clock defects observed in the lwd1 lwd2 mutant (Supplementary Fig. 1a). Only the model with LWD1 activating both PRR9 and CCA1 gave clock features resembling the short period and low amplitude of multiple clock genes in lwd1 lwd2 (ref. 14; Supplementary Fig. 1b). We also tested whether our modifications changed the general behaviours of the clock by adding cca1 lhy, toc1 or TOC1ox perturbations according to the settings in the original paper16. The addition of LWD1 activating both PRR9 and CCA1 in this model also maintained the perturbed clock behaviours similar to those reported previously16. These behaviours included the advanced phases of TOC1/EVENING COMPLEX and elevated levels of TOC1 mRNA in the cca1 lhy mutant (left panel in Supplementary Fig. 1c), and increased and decreased CCA1/LHY levels in toc1 and in TOC1ox, respectively (right panel in Supplementary Fig. 1c). Therefore, the integrity of the Pokhilko model could be maintained after incorporating LWD1 as an activator of both PRR9 and CCA1.
Our mathematical simulation provided a new hypothesis for LWD1 in clock regulation via CCA1 activation. We next examined whether LWD1 activated CCA1 by binding to the CCA1 promoter. Results of chromatin immunoprecipitation quantitative PCR (ChIP-qPCR) demonstrated a clear association of LWD1 with the CCA1 promoter at ZT21, preceding the peak expression of CCA1 (Fig. 1b), which implies the bona fide direct regulation of CCA1 by LWD1.
LWD1 interacts with TCP20 and TCP22
Although LWD1 has no recognizable DNA binding domain, it has 5 WD-repeats that constitute a propeller structure frequently considered a protein–protein interaction interface19. Therefore, LWD1 may be recruited to the CCA1 promoter by interacting with transcription factors that remain to be identified. We used yeast two-hybrid screening to identify LWD1-interacting transcription factor(s) from a prey library of ∼1,400 Arabidopsis full-length transcription factors20. TCP transcription factors were identified as potential interacting proteins of LWD1. An initial screen revealed four class I TCPs (TCP19, −20, −22 and CHE (also known as TCP21)) and TCP3 belonging to class II TCPs (Supplementary Table 1). TCP20 and TCP22 were the predominant interacting partners of LWD1 by their dominant presence (>98%) in the positive clones sequenced (Supplementary Table 1). Both LWD1 and LWD2 could interact with TCP20/TCP22 (Fig. 2a), which is consistent with the overlapping functions of LWD1 and LWD2 (ref. 21). Bimolecular fluorescence complementation assay (BiFC) confirmed that TCP20/TCP22 interacted with full-length LWD1 in Arabidopsis nuclei (Fig. 2b) but not truncated LWD1 with deletion of the fourth and fifth WD-repeats and expressed at a comparable level (Fig. 2b; Supplementary Fig. 2). The interactions between TCP20/TCP22 and LWD1 but not truncated LWD1 under the control of their native promoters were further validated in Arabidopsis seedlings with luciferase complementation imaging assays (Fig. 2c).
(a) LWD1/LWD2 and TCP20/TCP22 interact on yeast two-hybrid assay. Positive interactions between LWD1/LWD2 and TCP20/TCP22 were shown by the growth on the selection medium without Trp, Leu and His (SD-WLH+2 mM 3-AT) for the reporter gene HIS3. Bimolecular fluorescence complementation (BiFC) assay (b) and luciferase complementation imaging assay (c) showed that the co-expression between LWD1, but not truncated LWD1 (ΔLWD1), and TCP20/TCP22 reconstituted the activity of fluorescence protein (green signal) and luciferase. For luciferase complementation imaging (LCI) assay, one representative image from three independent is shown. Data are mean±s.e. (n=3). Asterisks indicate that LCI activity from the interactions of TCP20/TCP22 with LWD1 is significantly higher than that with ΔLWD1 (Student’s t test; *P<0.05). (d) Early flowering of tcp20-2, tcp22-1, and tcp20 tcp22 mutants under long-day conditions. Data are mean±s.d. (n≥10). Asterisks indicate that mutant plants flowered significantly earlier than wild-type plants (Student’s t test; *P<0.01). Ten to twelve plants for each genotype were planted for scoring for each biological replicate. Similar results were observed in three independent experiments. Scale bars, 10 μm.
Like other Arabidopsis short-period mutants3,5, the lwd1 lwd2 mutant has an early-flowering phenotype21. The protein–protein interaction between LWDs and TCP20/TCP22 suggested that TCP20/TCP22 might function with LWDs in flowering time control. Indeed, tcp20-2 (ref. 22), tcp20-4 (ref. 22) (Supplementary Fig. 3a), and tcp22-1 (ref. 23) single mutants and the tcp20 tcp22 double mutant show early transition from the vegetative to reproductive stage under long-day conditions (16-h light/8-h dark) (Fig. 2d and Supplementary Fig. 3b). The early-flowering phenotype of tcp mutants and the interaction between TCP and LWD proteins imply that TCP20/TCP22 also functions in the photoperiodic pathway.
TCP20 and TCP22 are new clock components
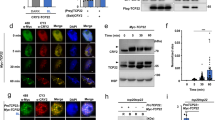
The expression of CCA1 is greatly dampened under constant dark condition21 and can be quickly induced by light signals24. To test whether TCP20 and TCP22 are required for CCA1 activation by light, seedlings of the wild type and tcp20 tcp22 were first grown under a 12-h light/12-h dark cycle for 10 days and then transferred to constant dark (DD) to minimize the CCA1 expression. The light-induced and rhythmic expression of CCA1 was triggered by 1 h of light treatment at DD24. The wild type showed peak CCA1 expression at DD45 (the following circadian cycle), but the expression amplitude was reduced in tcp20 tcp22 (Fig. 3a), which indicates that TCP20 and TCP22 are required for the full activation of CCA1 by light. We next investigated whether the activator role of TCPs on CCA1 expression results from a circadian equilibrium after a full 24-h rhythm or is a direct regulation by examining the immediate function of TCP20 on the CCA1 promoter. We fused TCP20 with the ligand-binding domain of glucocorticoid receptor (TCP20-GR) and expressed it under the control of a 35S promoter. With dexamethasone (DEX) treatment, TCP20-GR immediately enhanced the expression of the reporter gene luciferase (LUC2) driven by the CCA1 promoter in Arabidopsis seedlings (Fig. 3b), which implies a direct activator role of TCP20 on CCA1 expression.
(a) CCA1 transcript level is decreased in the tcp20 tcp22 mutant. Ten-d-old 12-h light/12-h dark grown seedlings were transferred to DD and illuminated by white light (75 μmol m−2 s−1) for 1 h at DD24. White, grey and black bars indicate light, subjective light and dark periods, respectively. DD, continuous dark. Data are mean±s.e. (n=3 technical replicates). An independent biological replicate showed similar result. Asterisks indicate that CCA1 transcript levels were significantly reduced in tcp20 tcp22 (Student’s t test; *P<0.005). (b) The nuclear transportation of TCP20-GR activated the expression of pCCA1(-984 to +1)::LUC2. Seedlings co-infected with Agrobacteria carrying 35S-TCP20-GR and pCCA1::LUC2 were released into constant light (LL) and treated with 40 μM dexamethasone (+DEX) or 0.4% EtOH (–DEX) at LL16 (arrow). Bioluminescent intensity at each time was normalized to that at LL14. Data are mean±s.d. from three biological replicates. Asterisks indicate that CCA1 promoter activity was significantly increased by DEX treatment (Student’s t test; *P<0.005). (c) The expression and period lengths of pCCA1::LUC2 are reduced in tcp20-2, tcp22-1 and tcp20 tcp22 mutants. Period length and relative amplitude error (RAE) were calculated by FFT-NLLS analysis according to data from LL48 to LL120 (n≥10 seedlings per genotype). Asterisks indicate that period lengths were significantly reduced in tcp mutants (Student’s t test; *P<0.01). (d) The expression of pTCP20::LUC2 and pTCP22::LUC2 is upregulated in the cca1 lhy mutant (n≥10 seedlings per genotype). (e) TCP20 and TCP22 transcript levels are increased in the cca1 lhy double mutant under short-day conditions. Data are mean±s.e. (n=3 technical replicates). Three independent biological replicates showed similar results. Asterisks indicate that TCP20 or TCP22 transcript levels were significantly increased in cca1 lhy (Student’s t test; *P<0.05).
To examine whether TCP20 and TCP22 regulate the circadian period length of CCA1, we introduced pCCA1::LUC2 into tcp single and double mutants by crossing with the reporter line in a wild-type background14. Consistent with the transcript levels, the expression of pCCA1::LUC2 was greatly reduced in tcp mutants (Fig. 3c and Supplementary Fig. 3c). Mutations in TCP20 or TCP22 shortened the period lengths by 0.7 h as compared with the wild type (Fig. 3c and Supplementary Fig. 3c). Under entrainment condition with a broadened morning peak, the expression of pCCA1::LUC2 was reduced in the tcp mutants as compared with the wild type (Supplementary Fig. 4). This finding is consistent with the short period phenotype, thus an advanced phase of CCA1 expression in tcp mutants. Of note, the short-period and early flowering phenotypes were not further exaggerated in the tcp20 tcp22 double mutant (Figs 3c and 2d). Therefore, TCP20 and TCP22 function cooperatively rather than redundantly in activating clock genes, possibly because TCP20 and TCP22 can exist as heterodimers25.
The low activity of the CCA1 promoter of tcp20-2 and tcp22-1 could be partially rescued by expressing pTCP20::TCP20-Flag and pTCP22::TCP22-Flag in the complementation lines, respectively (Supplementary Fig. 5a). The early-flowering phenotypes could also be complemented (Supplementary Fig. 5b). The degree of complementation was positively correlated with levels of TCP20-Flag or TCP22-Flag proteins in multiple independent lines (Supplementary Fig. 5c).
Like the activity of other clock genes identified previously, that of TCP20 and TCP22 promoters oscillated rhythmically under continuous light (LL) in the wild type (Fig. 3d). The transcript levels of TCP20 and TCP22 also oscillated slightly under the light/dark cycle (Fig. 3e). Both the promoter activities and transcript levels of TCP20 and TCP22 were enhanced in the cca1 lhy mutant (Fig. 3d,e), so the expression of TCP20/TCP22 was likely repressed by CCA1/LHY. Consistent with the repressor role of CCA1 on TCP20/TCP22, the peak CCA1 protein level at dawn4 coincided with the expression troughs of TCP20 and TCP22 (Fig. 3d,e). Thus, TCP20 and TCP22 are new clock genes under feedback regulation of the morning genes CCA1/LHY in the Arabidopsis circadian clock.
A canonical CCA1-binding site (CBS) and an evening element (EE) were found in the upstream sequences of TCP20 (−779 to −772) and TCP22 (−732 to −725), respectively, relative to the translational start site. We examined whether TCP20 and TCP22 are direct targets of CCA1 in the cca1-1 pCCA1-HA-gCCA1 complementation line. The complementation line accumulated a morning-phased HA-CCA1 protein as endogenous CCA1 (Supplementary Fig. 6a). ChIP-qPCR results showed clear binding of HA-CCA1 to the EE-like region (−549 to −543) in the CCA1 promoter and EE (−728 to −720) in the TOC1 promoter in vivo as reported26. However, HA-CCA1 did not seem to bind to TCP20 or TCP22 promoters (Supplementary Fig. 6b). Similarly, two recent genome-wide surveys of CCA1 target genes did not reveal TCP20 or TCP22 (refs 27, 28). Thus, CCA1 indirectly represses the expression of TCP20 and TCP22.
TCP20 and TCP22 directly activates the CCA1 promoter
CHE, also known as TCP21, represses CCA1 via TBS (GGTCCCAC, −564 to −557 relative to the translational start site)12, which is present in a region of the CCA1 promoter bound by LWD1 (Fig. 1b). We used ChIP-qPCR assays to examine whether TCP20/TCP22 associated with the TBS of the CCA1 promoter by using anti-Flag or TCP22-specific antisera with a tcp20-2 pTCP20::TCP20-Flag complementation line or wild-type plants. Only DNA fragment b containing TBS in the CCA1 promoter was enriched in the immunoprecipitated protein–chromatin complex from the complementation line (Fig. 4a) and wild-type plants (Supplementary Fig. 7). These results indicate a direct binding of TCP20/TCP22 to the CCA1 promoter in Arabidopsis.
(a) TCP20 associates with the TBS-containing region of CCA1 promoter in vivo. ChIP assays involved use of the anti-Flag antisera with tcp20-2 pTCP20::TCP20-Flag pCCA1::LUC2 complementation line (CL #20) or 35S::HA-GFP-Flag transgenic line. Data are mean±s.d. (n=3 technical replicates). Asterisk indicates that TCP20 preferentially binds with the amplicon ‘b’ (Student’s t test; *P<0.005). Similar results were observed in two independent experiments. (b) EMSA confirmed the specific binding of TCP20 and TCP22 with the TBS. DNA–protein complex was competed with excess (20 × or 40 ×) unlabelled wild-type (TBS) or mutated (mTBS) probes. (c) Transient assay in Arabidopsis protoplasts of TCP20 and TCP22 possessing activator activity toward promoters containing TBS but not mTBS. Effector and reporter constructs are illustrated. LUC2 reporter activity was normalized to GUS activity from the transfection control 35S::GUS. Data are mean±s.d. (n=3). Asterisks indicate that TCP20 or TCP22 preferentially activates the reporter gene driven by promoters containing TBS (mini35S was used as a negative control) (Student’s t test; *<0.005). Similar results were observed in three independent experiments.
Electrophoresis mobility shift assay showed that TCP20 and TCP22 could form a protein–DNA complex with a 50-bp fragment containing TBS (−584 to −535 of the CCA1 promoter relative to the translational start site) (Fig. 4b). As well, the formation of the TCP–TBS protein–DNA complex could be compromised only in the presence of excess and unlabelled TBS competitor but not the mutated TBS (mTBS) competitor (Fig. 4b), which suggests specific binding of TCP20/TCP22 to TBS in vitro. We further investigated the transcriptional activator role of TCP20 and TCP22 by binding to the TBS in plant cells. To simplify the regulation contributed by TBS, the same 50-bp CCA1 promoter fragment was used to assay the regulatory role of TCP20 and TCP22 on TBS in the protoplast transient system. The transcriptional activator activity of TCP20 and TCP22 relied on the presence of TBS in this promoter fragment (Fig. 4c). Consistently, the TCP20-dependent activation of the 634-bp CCA1 promoter was significantly compromised when TBS was mutated (Supplementary Fig. 8a). As expected, the activation was specific for the CCA1 promoter but not LHY promoter (Supplementary Fig. 8b), on which no putative TBS could be identified. Thus, TCP20 and TCP22 activate the expression of CCA1 via direct and specific binding to the TBS in the CCA1 promoter. For two functionally redundant genes such as CCA1 and LHY in the circadian clock, their differential regulation by TCPs may increase the flexibility and tunability of the circadian clock.
TCP20 and TCP22 activate CCA1 in an LWD1-dependent manner
Consistent with the activator roles of TCP20 and TCP22 on CCA1, CCA1 promoter activity was significantly greater in multiple independent transgenic lines overexpressing TCP20 (TCP20ox) or TCP22 (TCP22ox) than in wild-type plants (Fig. 5a and Supplementary Fig. 9a). To address whether interacting with LWDs was crucial for TCP20 and TCP22 to activate CCA1, we investigated the functional dependency of TCP20 and TCP22 on LWDs by comparing the CCA1 promoter activity in TCP20 or TCP22-overexpressing lines in the wild type or in lwd1 lwd2 backgrounds. Overexpression of TCP20 or TCP22 failed to enhance the CCA1 promoter activity in lwd1 lwd2 (Fig. 5b and Supplementary Fig. 9b), even though TCP20 and TCP22 levels were comparable in the wild type and lwd1 lwd2 (Supplementary Fig. 9c). Thus, TCP20 and TCP22 functioned as activators of CCA1 only in the presence of LWDs. Consistent with this notion, the trans-activation of TBS-containing promoter by TCP20 was significantly compromised in protoplasts from the lwd1 lwd2 mutant (Supplementary Fig. 10).
(a) pCCA1::LUC2 expression is increased in TCP20-overexpressing lines. (b) Overexpression of TCP20 fails to increase the expression of CCA1 promoter in lwd1 lwd2. Data are mean±s.e. (n=6–8 seedlings for each independent line; see the corresponding data for TCP22ox in Supplementary Fig. 9a,b). Similar results were observed in three independent experiments. (c) TCP20/TCP22 associate with the CCA1 promoter in vivo independent of LWDs. Asterisk indicates that overexpressed TCP20/TCP22 in WT or lwd1 lwd2 backgrounds preferentially binds with amplicon ‘b’ (Student’s t test; *P<0.005). (d) LWD1 associates with CCA1 promoter independent of TCP20/TCP22. Data are shown as mean±s.d. from one representative experiment (n=3 technical replicates). Asterisk indicates that LWD1 preferentially binds to the amplicon ‘b’ in both WT or tcp20 tcp22 (Student’s t test; *P<0.001). Similar results were observed in three independent experiments. (e) A diagram of LWD1 and TCP20/TCP22 co-activating the expression of CCA1.
We next examined whether this lack of CCA1 activation in lwd1 lwd2 was due to a compromised binding of TCP20 and TCP22 to the TBS in the CCA1 promoter. TCP20 and TCP22 could still associate with the TBS in the CCA1 promoter with or without LWDs (Fig. 5c). Our results also showed comparable association of LWD1 with the TBS in the CCA1 promoter in the wild type and tcp20 tcp22 double mutant (Fig. 5d), so LWD1 could still be recruited to the CCA1 promoter in tcp20 tcp22, possibly via other transcription factors. In-depth yeast two-hybrid assays revealed additional LWD1- and LWD2-interacting class I TCPs, including TCP7, TCP8, TCP9, TCP14, TCP15 and TCP23 (Supplementary Fig. 11a), which were distributed in various clades in a phylogenetic tree of class I TCPs (Supplementary Fig. 11b). Among the members in the same clade containing TCP20 and TCP22, TCP14 and TCP23 could also activate CCA1 expression in a protoplast transient assay (Supplementary Fig. 11c), which suggests that additional class I TCPs have activator roles for CCA1. This finding may explain why the expression of CCA1 was not completely compromised in tcp20 tcp22 (Fig. 3a,c).
LWD1 protein accumulates throughout the day (Supplementary Fig. 12), thereby rendering accessibility in interacting with both activators (TCP14, TCP20, TCP22 and TCP23) and the repressor (CHE; Supplementary Table 1) of CCA1 in the TCP superfamily. Future in-depth studies of LWDs are needed to uncover their regulatory roles at different times of the day via different interacting transcription factors and/or target genes.
Collectively, although TCP20/TCP22 could directly target the TBS in the CCA1 promoter, the binding of TCP20/TCP22 to the CCA1 promoter was insufficient for CCA1 activation without the concomitant binding of LWDs. The interaction with LWDs likely confers TCP20/TCP22 with transcriptional activator activity. Therefore, LWDs are the co-activators of TCP20 and TCP22 for the transcriptional activation of CCA1 (left panel in Fig. 5e).
Discussion
Transcriptional oscillation can be steadily produced by the combination of three repressors in a repressilator system29; however, the amplitude and robustness of biological oscillations could be better maintained when the system contains interlocked positive and negative feedback loops13. In the Arabidopsis circadian clock, LWD1 and PRR9/PRR7 constitute a positive feedback loop14. The expression of the clock co-activator LWD1 was significantly reduced in prr9 and prr7 mutants14. Without the full capacity of LWD1 as the co-activator, CCA1 expression amplitude at dawn could be not maintained, which explains the much-reduced CCA1 expression peak in the higher-order CCA1 repressor mutants (prr9 prr7 and prr9 prr7 prr5 toc1)30.
Previous mathematical modelling of clock gene expression in Arabidopsis was mostly based on repressors in the negative regulatory loops16,31. The regulation of CCA1 expression revealed only transcriptional repressors, including PRR9, PRR7, PRR5, TOC1 and CHE. Of note, CHE represses CCA1 by binding to TBS (ref. 12). We provide direct evidence that the LWD–TCP complex could activate and maintain the robust rhythm of CCA1 by also binding to TBS. Thus, TCP family members could positively or negatively regulate CCA1, and TBS is a target for both negative (CHE) and positive (TCP20 and TCP22) regulators.
Although LNKs function as co-activators of RVEs for the expression of PRR5 and TOC1 (ref. 15), they can antagonize RVEs’ positive regulation of anthocyanin biosynthesis genes at a specific time during the day32, which indicates a time-dependent switch of roles of LNKs on RVEs toward different target genes. The consecutive expression of the repressors PRR9, PRR7, PRR5, TOC1 and CHE (refs 8, 9, 10, 11, 12, 16), followed by the activators TCP20/TCP22, during a 24-h cycle likely shapes a precise expression peak of CCA1 at dawn (right panel in Fig. 5e). Whether the interaction between LWDs and CHE (Supplementary Table 1 and Supplementary Fig. 13) implies a regulatory role of LWDs for CHE remains to be addressed. Additional work is needed to decipher the complex regulation of the Arabidopsis circadian clock by TCP transcription factors.
Methods
Plant materials and growth conditions
The wild type and clock mutants lwd1 lwd2, cca1-1 and lhy-101 in Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used for all experiments21,33,34. The cca1 lhy double mutant was generated by crossing cca1-1 and lhy-101 mutants. T-DNA insertion lines tcp20-2 (SALK_088460c), tcp20-4 (SALK_041906c) and tcp22-1 (SALK_027490) were obtained from the Arabidopsis Biological Resources Center. The tcp20 tcp22 double mutant was generated by crossing tcp20-2 and tcp22-1 mutants. For qRT-PCR experiments shown in Fig. 3a, 10-d-old seedlings grown on 0.8% phytoagar solidified half-strength MS (1/2 MS) medium under 12-h light/12-h dark conditions (75 μmol m−2 s−1) were transferred to constant dark. In Fig. 3e, 18-d-old seedlings were grown under 8-h light/16-h dark conditions (75–100 μmol m−2 s−1).
Chromatin immunoprecipitation quantitative PCR assay
To detect direct target genes of endogenous LWD1 or TCP22, chromatin immunoprecipitation quantitative PCR (ChIP-qPCR) assays were performed by using 18-d-old wild-type, lwd1 lwd2 or tcp20 tcp22 mutants grown under 16-h light/8-h dark on half-strength MS medium. Tissues were harvested at the indicated ZT points. Four hundred plants were treated with 35 ml cross-linking buffer (0.4 M sucrose, 10 mM Tris–HCl, pH 8.0 and 1% formaldehyde) under vacuum for four times, each with 5 min. Glycine was added to a final concentration of 125 mM to quench the excess formaldehyde and to terminate the cross-linking reaction under vacuum for twice, each for 2.5 min, followed by rinsing with 50 ml deionized water for three times. The plants were briefly dried and ground to powder in liquid nitrogen. Five hundred μl powder was then lysed in 0.8 ml cold nuclei lysis buffer (50 mM HEPES pH 7.5, 150 mM NaCl, 0.5% SDS, 0.1% sodium deoxycholate, 1% Triton X-100, 0.1 mM PMSF, 50 μM MG115 and 1 × protease inhibitor cocktail (Roche, Monnheim, Germany)). The lysate was filtered through 100 μm nylon mesh (Calbiochem, La Jolla, CA) in a 2 ml centrifuge tube, and sonicated in a ice bath with the use of Bioruptor (Diagenode, Liege, Belgium) set at high power and 15-s ON/15-s OFF for seven times, each with 5 min. A one-tenth volume of the sonicated lysate was saved for the input fraction control. The LWD1 or TCP22-associated chromatin complexes were immunoprecipitated by incubating the lysate with 3 μl rabbit polyclonal antisera generated with recombinant LWD1 (amino acid residues 1–73) or a polypeptide comprising residues 361–375 (PNQSQASENGGDDKK) of TCP22 for overnight at 4 °C. The immunoprecipitated chromatin complexes were purified by incubating with 50 μl slurry of nProtein A Sepharose (GE, 17-5280-01) pre-equilibrated with 1 mg ml−1 salmon sperm DNA and 1 mg ml−1 BSA for 3 h at 4 °C.
For HA-tagged CCA1-ChIP, a DNA fragment encoding three tandem repeats of HA-tag was inserted in front of the start codon of a CCA1 genomic fragment spanning from −1,418 (relative to the translational start site) to the stop codon to generate the CCA1 complementation construct into BamHI- and KpnI-digested pCAMBIA1390. For Flag–tagged TCP20 or TCP22-ChIP, the overexpressing lines were generated by inserting the coding region of TCP20 or TCP22 into NcoI and BamHI sites of a modified version of pEarleyGate100 (ref. 35) to generate CaMV35S (35S) promoter-driven Flag-TCP20 or Flag-TCP22 under wild-type or lwd1 lwd2 background. ChIP analyses were performed by using 50 μl Sigma monoclonal anti-HA agarose antibody (A2095) or Sigma monoclonal anti-Flag M2 affinity gel (A2225) for HA-CCA1 or Flag-TCP20 and Flag-TCP22.
The affinity beads bound chromatin complexes were washed with 850 μl nuclei lysis buffer for three times, LNDET buffer (0.25 M LiCl, 1% NP40, 1% sodium deoxycholate, 1 mM EDTA, pH8.0) for three times and TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH8.0) for three times. Chromatin complexes were eluted from the beads with use of 200 μl elution buffer (1% SDS, 0.1 M NaHCO3) followed by the digestion with 2.5 μl Proteinase-K (20 mg ml−1, Invitrogen, Carlsbad, CA) for overnight at 65 °C. DNA released from chromatin complexes was then purified by use of a QIAEXII gel purification kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instruction. Primer sequences used for PCR amplification are listed in Supplementary Table 2. Independent biological replicates of ChIP-qPCR results are shown in Supplementary Fig. 14.
Yeast two-hybrid analysis
LWD1-interacting proteins were screened in a prey library of ∼1,400 transcription factors by yeast two-hybrid assay20. Protein–protein interactions were re-confirmed by using a GAL4-based yeast two-hybrid system (MATCHMAKER GAL4 two-hybrid system, Clontech). Empty pDBLeu (Invitrogen) and pEXP-AD502HA plasmids were used as negative bait and prey controls, respectively. The coding region of LWD1 or LWD2 was cloned into the XmaI and NotI sites of pDBLeu. The pEXP-AD502HA vector is a modified version of the pEXP-AD502 (Invitrogen) plasmid. Briefly, the T7-HA-MCS derived from pGADT7 (Clontech) was inserted into the SalI and NotI sites of the pEXP-AD502 vector. Coding regions of TCP6, 7, 8, 9, 11, 14, 15, 16, 20, 22 and 23 were cloned into pEXP-AD502HA. SD-WL (-Trp -Leu) plates were used to select for the presence of both bait and prey vectors, and SD-WLH (-Trp -Leu -His) plates with 3-AT (3-amino-1, 2, 4-triazole) were used to select for interactions between the bait and prey proteins. All primers for these constructs for yeast two-hybrid assays are in Supplementary Table 2.
Bimolecular fluorescence complementation (BiFC) assay
The LWD1 full-length coding region or truncated variant lacking amino acids 236–290 (ΔLWD1) was subcloned into hygII-VYNE(R)-pGPTVII, and the coding regions of TCP20 or TCP22 were subcloned into kanII-SCYCE(R)-pGPTVII (ref. 36). All primers of these constructs for BiFC are in Supplementary Table 2. VenusN173-fused LWD1 or ΔLWD1 was transiently co-expressed with SCFP3AC155-fused TCP20, TCP22 or CHE in Arabidopsis seedlings by the AGROBEST method37. Three days after infection, a GFP filter set was used for detecting the fluorescence signals of SCFP3AC and VenusN by Zeiss LSM 780 confocal microscopy.
Luciferase complementation imaging assay
Luciferase complementation imaging assays38 were performed with constructs pLWD1::NLUC2-LWD1, pLWD1::NLUC2-ΔLWD1, pTCP20::CLUC2-TCP20 and pTCP22::CLUC2-TCP22 in Arabidopsis seedlings by AGROBEST37. Coding regions of LWD1 (ΔLWD1) and TCP20/TCP22 were fused with firefly luciferase NLUC2 (+1 to +1,248, relative to the translational start site) and CLUC2 (+1,194 to +1,650), respectively, and expressed under the control of their native promoters, LWD1 (−1,068 to −1), TCP20 (−2,564 to −1), and TCP22 (−2,000 to −1).
Flowering time determination
Arabidopsis seeds were sown on soil and stratified for 3 or 4 days at 4 °C, and then grown under long-day (16-h light/8-h dark) conditions at fluence rate 80–100 μmol m−2 s−1 at 22 °C. The number of rosette leaves ≥ 5 mm long was recorded for each plant when the primary florescence reached 5 cm above the rosette leaves. This phenotype observation was repeated at least three times.
RNA preparation and quantitative real-time PCR
Plants grown under conditions/ZT indicated were harvested and ground in liquid nitrogen. Total RNA was extracted by mixing the tissue powder from 10 plants with 700 μl of 65 °C pre-warmed pine-tree buffer (2% hexadecyltrimethylammonium, 2% polyvinylpyrrolidone K30, 100 mM Tris–HCl pH 8.0, 25 mM EDTA, 2 M NaCl, 0.5 g l−1 spermidine, freshly added 2% β-mercaptoethanol)39. The extraction mixture was incubated at 65 °C for 5 min. After mixing with 700 μl of chloroform: isoamyl alcohol (24:1), the extraction mixture was centrifuged for 20 min at room temperature. The RNA in the liquid phase was precipitated overnight at 4 °C in a final concentration of 2 M LiCl. RNA pellet was washed by 70% ethanol, briefly air dried, and suspended in DEPC treated mili Q water. Quantitative real-time RT-PCR (qRT-PCR) was performed by using the Applied Biosystem QuantStudio 12K Flex Real-Time PCR System according to the manufacturer’s instructions. Sequences and quantities of primers for each gene are listed in Supplementary Table 2. The comparative CT method was used to determine the relative gene expression, with the expression of UBQ10 or UBC21 as the internal control. Mean values of 2−ΔCT (ΔCT=CT, gene of interest–CT, UBQ10 or UBC21) were calculated from three technical repeats. Independent biological replicates of qRT-PCR results are shown in Supplementary Fig. 14.
Bioluminescence measurement and data analyses
The coding region of TCP20 without a stop codon was fused to the ligand-binding domain of glucocorticoid receptor to generate a TCP20-GR effector construct driven by a 35S promoter with modified pEarleyGate100. A 984-bp DNA fragment of CCA1 promoter (−984 to −1 relative to the translational start site) was cloned and inserted into PstI and NcoI sites to fuse with the LUC2 gene in the binary vector pCAMBIA1390 (CSIRO, Australia) for constructing the pCCA1(−984)::LUC2 reporter. The effector and reporter constructs were used in the AGROBEST system for transient expression in Arabidopsis seedlings under long-day conditions as described37. Ten infected seedlings were used for each of three biological replicates for independent induction by 40 μM dexamethasone or 0.4% ethanol mock at the indicated times in bioluminescence analyses.
The pCCA1::LUC2 reporter line for CCA1 promoter region from −1,418 to −1 relative to the translational start site was used14. To generate the pTCP20::LUC2 and pTCP22::LUC2 reporter lines, the upstream 2564- and 2000-bp fragments of the translational start sites for TCP20 and TCP22, respectively, were cloned into the PstI and SalI sites to fuse with the LUC2 gene in pCAMBIA1390. Seven-d-old seedlings were grown 1/2 MS medium under 16-h light/8-h dark (75 μmol m−2 s−1), then transferred to continuous light (30–35 μmol m−2 s−1) at ZT0. Seedlings were imaged every 1 h for 5 d. Bioluminescence activity was measured14. Period length was calculated by using the Biological Rhythms Analysis Software System (BRASS40, available at http://www.amillar.org) and analysed with use of fast Fourier transform-non linear least squares (FFT-NLLS).
Electrophoretic mobility shift assay
The coding region of TCP20 or TCP22 was cloned into the pET42a (+) vector (Novagen) to generate GST-tagged TCP20 or TCP22. Recombinant GST-TCP20 and GST-TCP22 were expressed in Escherichia coli BL21(DE3) and purified by using glutathione-affinity agarose beads (GE) following the manufacturer’s protocol. To generate radioactive probes used in electrophoretic mobility shift assay (EMSA), two oligonucleotides for the wild-type or mutated TBS sequence with 10-nt complementary 3′-ends were annealed at a final concentration of 50 μM for each oligonucleotide in TE buffer. The partially annealed DNA were filled-in and radiolabeled for 30 min at 37 °C in a 10 μl reaction mixture comprised 25 μCi of (α-32P)dCTP (specificity equals to 3,000 Ci per mmol; Perkin-Elmer), 2.5 unit Klenow fragment of DNA polymerase I (New England BioLabs), labelling buffer (200 mM HEPES, 50 mM Tris–HCl pH8.0, 5 mM MgCl2, 10 mM β-mercaptoethanol, 20 μM for each of dATP, dTTP and dGTP). The oligonucleotides used to generate radiolabeled DNA probes and unlabelled competitors are listed in Supplementary Table 2. EMSA was performed41 by mixing 0.05 pmol of the probe with purified recombinant protein in the binding buffer (35 mM KCl, 15 mM HEPES 7.5, 0.4 mM EDTA, 6% glycerol, 50 ng μl−1 poly(dI-dC)) with or without competitors at room temperature for 10 min. DNA–protein complexes were separated by a 4.5% Tris/Borate/EDTA polyacrylamide gel under 0.5 × Tris-Glycine buffered electrophoresis system, and fixed in 10% acetic acid for autoradiography detection.
Transient expression in Arabidopsis protoplasts
DNA fragments relative to the translational start site of CCA1 including 50-bp (−584 to −535) fused with 35S minimum promoter and 634-bp (−634 to −1) of the CCA1 promoter containing the wild-type or mutated TCP binding site (TBS or mTBS, respectively), and LHY promoter (−1,661 to −1) (ref. 14) were ligated to the upstream of LUC2 in the LUC2-modified pJD30142 vector to generate reporter constructs in protoplasts. The coding regions of GFP, LWD1, TCP14, TCP20, TCP22 or TCP23 were tagged with epitope HA and sub-cloned into the p326-GFP (ref. 43) vector for transient overexpression under the control of the 35S promoter. For transient assay in Arabidopsis protoplasts, 150 leaves of 3-wk-old plants grown under 12-h light/12-h dark condition were to isolate mesophyll protoplasts by the sandwich method. Briefly, the leaves were digested in the enzyme solution (1% cellulase R10, 0.25% macerozyme R10, 0.4 M mannitol, 20 mM KCl, 20 mM MES, pH5.7, 10 mM CaCl2, 5 mM β-mercaptoethanol, 0.1% BSA) to obtain 2.5 × 106 mesophyll protoplasts for transfections44,45. An amount of 2.5 × 105 protoplasts was co-transfected with 2 μg reporter construct, 5 μg 35S::TCP(s)-HA as the effector and 10 μg pBI221 (35S::GUS) as the transfection control. Transfected cells were incubated at 22 °C overnight and harvested for LUC2 and GUS reporter assays.
Phylogenic analysis
The phylogeny analysis of full-length protein sequences class I TCP members was performed with the one-click mode of the web-based phylogeny tool Phylogeny.fr (http://www.phylogeny.fr/)46. Sequence data for genes referred to in this study can be found in the Arabidopsis Genome Initiative data library with the following locus identifiers: LWD1 (At1g12910), LWD2 (At3g26640), CCA1 (At2g46830), LHY (At1g01060), PRR7 (At5g02810), PRR9 (At2g46790), TOC1 (At5g61380), TCP3 (At1g53230), TCP6 (At5g41030), TCP7 (At5g23280), TCP8 (At1g58100), TCP9 (At2g45680), TCP11 (At2g37000), TCP14 (At3g47620), TCP15 (At1g69690), TCP16 (At3g45150), TCP19 (At5g51910), TCP20 (At3g27010), TCP21/CHE (At5g08330), TCP22 (At1g72010), TCP23 (At1g35560), UBQ10 (At4g05320) and UBC21 (At5g25760).
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its Supplementary Information Files or are available from the corresponding author upon request.
Additional information
How to cite this article: Wu, J.-F. et al. LWD–TCP complex activates the morning gene CCA1 in Arabidopsis. Nat. Commun. 7, 13181 doi: 10.1038/ncomms13181 (2016).
References
Dodd, A. N. et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309, 630–633 (2005).
Alabadi, D. et al. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293, 880–883 (2001).
Strayer, C. et al. Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science 289, 768–771 (2000).
Wang, Z. Y. & Tobin, E. M. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93, 1207–1217 (1998).
Mizoguchi, T. et al. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev. Cell 2, 629–641 (2002).
McClung, C. R. Wheels within wheels: new transcriptional feedback loops in the Arabidopsis circadian clock. F1000Prime Rep. 6, 2 (2014).
Somers, D. E. The Arabidopsis clock: time for an about-face? Genome Biol. 13, 153 (2012).
Gendron, J. M. et al. Arabidopsis circadian clock protein, TOC1, is a DNA-binding transcription factor. Proc. Natl Acad. Sci. USA 109, 3167–3172 (2012).
Huang, W. et al. Mapping the core of the Arabidopsis circadian clock defines the network structure of the oscillator. Science 336, 75–79 (2012).
Nakamichi, N. et al. PSEUDO-RESPONSE REGULATORS 9, 7, and 5 are transcriptional repressors in the Arabidopsis circadian clock. Plant Cell 22, 594–605 (2010).
Nakamichi, N., Kita, M., Ito, S., Yamashino, T. & Mizuno, T. PSEUDO-RESPONSE REGULATORS, PRR9, PRR7 and PRR5, together play essential roles close to the circadian clock of Arabidopsis thaliana. Plant Cell Physiol. 46, 686–698 (2005).
Pruneda-Paz, J. L., Breton, G., Para, A. & Kay, S. A. A functional genomics approach reveals CHE as a component of the Arabidopsis circadian clock. Science 323, 1481–1485 (2009).
Tsai, T. Y. et al. Robust, tunable biological oscillations from interlinked positive and negative feedback loops. Science 321, 126–129 (2008).
Wang, Y. et al. LIGHT-REGULATED WD1 and PSEUDO-RESPONSE REGULATOR9 form a positive feedback regulatory loop in the Arabidopsis circadian clock. Plant Cell 23, 486–498 (2011).
Xie, Q. et al. LNK1 and LNK2 are transcriptional coactivators in the Arabidopsis circadian oscillator. Plant Cell 26, 2843–2857 (2014).
Pokhilko, A. et al. The clock gene circuit in Arabidopsis includes a repressilator with additional feedback loops. Mol. Syst. Biol. 8, 574 (2012).
Gould, P. D. et al. Network balance via CRY signalling controls the Arabidopsis circadian clock over ambient temperatures. Mol. Syst. Biol. 9, 650 (2013).
Seaton, D. D. et al. Linked circadian outputs control elongation growth and flowering in response to photoperiod and temperature. Mol. Syst. Biol. 11, 776 (2015).
Smith, T. F., Gaitatzes, C., Saxena, K. & Neer, E. J. The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 24, 181–185 (1999).
Kim, S. H. et al. The Arabidopsis immune adaptor SRFR1 interacts with TCP transcription factors that redundantly contribute to effector-triggered immunity. Plant J. Cell Mol. Biol. 78, 978–989 (2014).
Wu, J. F., Wang, Y. & Wu, S. H. Two new clock proteins, LWD1 and LWD2, regulate Arabidopsis photoperiodic flowering. Plant Physiol. 148, 948–959 (2008).
Guan, P. et al. Nitrate foraging by Arabidopsis roots is mediated by the transcription factor TCP20 through the systemic signaling pathway. Proc. Natl Acad. Sci. USA 111, 15267–15272 (2014).
Aguilar-Martinez, J. A. & Sinha, N. Analysis of the role of Arabidopsis class I TCP genes AtTCP7, AtTCP8, AtTCP22, and AtTCP23 in leaf development. Front. Plant Sci. 4, 406 (2013).
Kikis, E. A., Khanna, R. & Quail, P. H. ELF4 is a phytochrome-regulated component of a negative-feedback loop involving the central oscillator components CCA1 and LHY. Plant J. cell Mol. Biol. 44, 300–313 (2005).
Danisman, S. et al. Arabidopsis class I and class II TCP transcription factors regulate jasmonic acid metabolism and leaf development antagonistically. Plant Physiol. 159, 1511–1523 (2012).
Adams, S., Manfield, I., Stockley, P. & Carre, I. A. Revised morning loops of the Arabidopsis circadian clock based on analyses of direct regulatory interactions. PLOS ONE 10, e0143943 (2015).
Nagel, D. H. et al. Genome-wide identification of CCA1 targets uncovers an expanded clock network in Arabidopsis. Proc. Natl Acad. Sci. USA 112, E4802–E4810 (2015).
Kamioka, M. et al. Direct repression of evening genes by CIRCADIAN CLOCK-ASSOCIATED 1 in Arabidopsis circadian clock. Plant Cell 28, 696–711 (2016).
Elowitz, M. B. & Leibler, S. A synthetic oscillatory network of transcriptional regulators. Nature 403, 335–338 (2000).
Yamashino, T. et al. Involvement of Arabidopsis clock-associated pseudo-response regulators in diurnal oscillations of gene expression in the presence of environmental time cues. Plant Cell Physiol. 49, 1839–1850 (2008).
Pokhilko, A., Mas, P. & Millar, A. J. Modelling the widespread effects of TOC1 signalling on the plant circadian clock and its outputs. BMC Syst. Biol. 7, 23 (2013).
Perez-Garcia, P., Ma, Y., Yanovsky, M. J. & Mas, P. Time-dependent sequestration of RVE8 by LNK proteins shapes the diurnal oscillation of anthocyanin biosynthesis. Proc. Natl Acad. Sci. USA 112, 5249–5253 (2015).
Khanna, R. et al. Functional profiling reveals that only a small number of phytochrome-regulated early-response genes in Arabidopsis are necessary for optimal deetiolation. Plant Cell 18, 2157–2171 (2006).
Yakir, E. et al. Posttranslational regulation of CIRCADIAN CLOCK ASSOCIATED1 in the circadian oscillator of Arabidopsis. Plant Physiol. 150, 844–857 (2009).
Earley, K. W. et al. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. Cell Mol. Biol. 45, 616–629 (2006).
Waadt, R. et al. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J.: Cell Mol. Biol. 56, 505–516 (2008).
Wu, H. Y. et al. AGROBEST: an efficient Agrobacterium-mediated transient expression method for versatile gene function analyses in Arabidopsis seedlings. Plant Methods 10, 19 (2014).
Chen, H. et al. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 146, 368–376 (2008).
Chang, S., Puryear, J. & Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Reporter 11, 113–116 (1993).
Southern, M. M., Brown, P. E. & Hall, A. Luciferases as reporter genes. Methods Mol. Biol. 323, 293–305 (2006).
Wang, H., Tang, W., Zhu, C. & Perry, S. E. A chromatin immunoprecipitation (ChIP) approach to isolate genes regulated by AGL15, a MADS domain protein that preferentially accumulates in embryos. Plant J. Cell Mol. Biol. 32, 831–843 (2002).
Luehrsen, K. R., de Wet, J. R. & Walbot, V. Transient expression analysis in plants using firefly luciferase reporter gene. Methods Enzymol. 216, 397–414 (1992).
Lee, Y. J., Kim, D. H., Kim, Y. W. & Hwang, I. Identification of a signal that distinguishes between the chloroplast outer envelope membrane and the endomembrane system in vivo. The Plant cell 13, 2175–2190 (2001).
Wu, F. H. et al. Tape-Arabidopsis Sandwich - a simpler Arabidopsis protoplast isolation method. Plant Methods 5, 16 (2009).
Yoo, S.-D., Cho, Y.-H. & Sheen, J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protocols 2, 1565–1572 (2007).
Dereeper, A. et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36, W465–W469 (2008).
Acknowledgements
We thank Jen Sheen and the Wu lab members for helpful discussion. This research was supported by an Academia Sinica postdoctoral fellowship to H.-L.T., research grants from the Ministry of Science and Technology, Taiwan, to S.-H.W. (100-2311-B-001-028-MY3 and 103-2311-B-001-009-MY3), C.-P.H. (104-2627-M-001-003- and 103-2627-M-001-004-), grants from the Next Generation Biogreen 21 Program (SSAC grant PJ01107102) and the Basic Science Research Program funded by the Ministry of Education (NRF- 2013R1A1A2010131) to J.C.H.
Author information
Authors and Affiliations
Contributions
J.-F.W., H.-L.T., I.J., C.-P.H. and S.-H.W. designed the research, analysed the data and wrote the article. J.-F.W., H.-L.T., I.J., Y.-C.W., Y.-H.L. and C.-W.C. performed the research. J.-W.C. and C.-P.H. supervised the computational work. Y.W. and J.C.H. contributed experimental materials.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-14, Supplementary Tables 1-4, Supplementary Note 1 and Supplementary References. (PDF 3244 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wu, JF., Tsai, HL., Joanito, I. et al. LWD–TCP complex activates the morning gene CCA1 in Arabidopsis. Nat Commun 7, 13181 (2016). https://doi.org/10.1038/ncomms13181
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms13181
This article is cited by
-
Unlocking allelic variation in circadian clock genes to develop environmentally robust and productive crops
Planta (2024)
-
Genome-wide screening and characterization of long noncoding RNAs involved in flowering/bolting of Lactuca sativa
BMC Plant Biology (2023)
-
Population genomics identifies genetic signatures of carrot domestication and improvement and uncovers the origin of high-carotenoid orange carrots
Nature Plants (2023)
-
Genome-wide TCP transcription factors analysis provides insight into their new functions in seasonal and diurnal growth rhythm in Pinus tabuliformis
BMC Plant Biology (2022)
-
Arabidopsis cryptochrome 2 forms photobodies with TCP22 under blue light and regulates the circadian clock
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.