Abstract
Intestinal tumour formation is generally thought to occur following mutational events in the stem cell pool. However, active NF-κB signalling additionally facilitates malignant transformation of differentiated cells. We hypothesized that genes shared between NF-κB and intestinal stem cell (ISCs) signatures might identify common pathways that are required for malignant growth. Here, we find that the NF-κB target Bcl-2, an anti-apoptotic gene, is specifically expressed in ISCs in both mice and humans. Bcl-2 is dispensable in homeostasis and, although involved in protecting ISCs from radiation-induced damage, it is non-essential in tissue regeneration. Bcl-2 is upregulated in adenomas, and its loss or inhibition impairs outgrowth of oncogenic clones, because Bcl-2 alleviates apoptotic priming in epithelial cells following Apc loss. Furthermore, Bcl-2 expression in differentiated epithelial cells renders these cells amenable to clonogenic outgrowth. Collectively, our results indicate that Bcl-2 is required for efficient intestinal transformation following Apc-loss and constitutes a potential chemoprevention target.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide, with ∼1.2 million new diagnoses and over 600,000 fatalities each year1. Adequate treatment is challenging and the development of new preventive and therapeutic strategies remains crucial. CRC formation is a stepwise process during which various histopathological stages can be detected2. Each stage is characterized by an accumulation of genetic aberrations that provide a competitive advantage to clones that arise following their occurrence3,4. Previously it has been reported that transformation occurs most efficiently following mutation in the intestinal stem cell (ISC) compartment5. For example, activation of the Wnt pathway, commonly arising following inactivation of the Apc gene, specifically in ISCs rapidly results in tumour formation, whereas, Apc inactivation in more differentiated cells fails to induce tumour growth5,6,7,8. This suggests that ISCs are the cell of origin of a large proportion of CRCs. Critically, the molecular mechanism by which ISCs more readily generate pre-neoplastic expansions on acquisition of mutations than their differentiated descendants is unknown. We hypothesized that identifying the mediators of ISC susceptibility to transformation might suggest potentially attractive chemopreventive and therapeutic targets.
Clues to identify signals associated with CRC development might be derived from conditions that affect the risk of onset of the disease. For example, inflammatory bowel disease is associated with an increased susceptibility to develop CRC9. Reciprocally, usage of compounds with anti-inflammatory properties such as non-steroidal anti-inflammatory drugs, including celecoxib and aspirin, are associated with a small, but significant reduction in respectively colorectal adenoma and carcinoma incidence and likely impact on tumour initiation10,11,12. Moreover, intestinal inflammation has recently been shown to increase the pool of cells susceptible to oncogenic transformation to include intestinal differentiated cells (IDC)7,8. Specifically, activation of the NF-κB pathway in differentiated cells, which is an important mediator of the intestinal inflammatory response, renders these cells capable of generating tumours on inactivation of the Apc gene7. Conversely, genetic inactivation of the NF-κB pathway, either directly by p65-inactivation or by reducing reactive oxygen species following Rac1-loss, resulted in a reduced tumour incidence, further suggesting a critical role for this pathway in CRC genesis13,14.
Here we report that ISCs exhibit an intrinsic activation of the NF-κB pathway. Moreover, we show that B-cell lymphoma 2 (Bcl-2), a key NF-κB target gene, is highly expressed in ISCs. Genetic as well as pharmacological inactivation of BCL-2 impairs adenoma formation and results in formation of indolent lesions similar to cyst-like structures observed when oncogenic mutations are introduced in the differentiated cell pool. Furthermore, Bcl-2 overexpression in differentiated cells generates clonogenic cells on Apc-loss. Our data thus reveals that Bcl-2 is an important mediator of transformation of intestinal epithelial cells but its loss bears no impact on the intestine in homeostasis. Therefore future studies are warranted to explore if intestine-specific BCL-2 inhibition could be used as a chemopreventive strategy for CRC.
Results
ISCs display high NF-κB activity and target gene expression
Intestinal epithelium transformation occurs most efficiently in the ISC compartment5. As NF-κB signalling has been shown to be a critical component for transformation to occur, we investigated whether NF-κB signalling activity was increased in the ISC compartment7,8. Gene set enrichment analysis (GSEA) revealed an increased expression of NF-κB target genes in Lgr5high versus Lgr5low cells (Fig. 1a). Moreover, when compared with neighbouring Paneth cells and other differentiated cells, increased NF-κB pathway activity was observed in the crypt base, where the ISCs reside, as evidenced by nuclear accumulation of RELA/p65 (Fig. 1b). To identify additional genes required for the transformation of NF-κBlow IDCs but not NF-κBhigh ISCs, we identified NF-κB target genes that are most differentially expressed between ISCs (Lgr5-GFPhigh, EphB2high) and IDCs (Lgr5-GFPlow, EphB2low). This revealed five genes: Ncl, Cd44, Cep110, Bub1b and Bcl-2, to be highly significantly (P<0.01) differentially expressed in ISCs versus IDCs in various public data sets (Fig. 1c; Supplementary Fig. 1)15,16. Because Bcl-2 is a gene that is well known for its anti-apoptotic properties and the only gene we identified that can be effectively pharmacologically inhibited, we focused our attention on this candidate. To confirm that Bcl-2 is an NF-κB target gene in intestinal epithelial cells we treated organoid cultures with TNFα to enhance NF-κB activity, as well as with an NF-κB inhibitor (JSH-23), resulting in increased and decreased Bcl-2 messenger RNA levels respectively (Fig. 1d,e). Furthermore, we observed an increase in p65 binding to the Bcl-2 promoter on TNFα stimulation of intestinal epithelial cells (Fig. 1f,g; Supplementary Fig. 10). Thus, Bcl-2 is a direct NF-κB target within the intestinal epithelium.
(a) GSEA demonstrates higher NF-κB target gene expression in Lgr5-GFPhigh versus Lgr5-GFPlow cells. ES, enrichment score; NES, normalized enrichment score. The NF-κB signature was adapted from Compagno et al. (n=120 genes)48. (b) Immunohistochemistry for RELA in murine small intestinal tissue. Nuclear staining of RELA indicates a high activity of the NF-κB pathway in the ISC compartment compared with the differentiated cell lineages. Scale bar, 100 μm. (c) Venn diagram indicating the significantly (P<0.01) differentially expressed genes for the indicated conditions (left and right circle) and genes listed in the gene category ‘drug targets’ (bottom circle) of the NF-κB pathway within the NF-κB target gene signature. (a,c) Gene expression profiles of Lgr5-GFPhigh/low and EphB2high/low are obtained from GSE33948 and GSE27605, respectively. (d) Treatment of intestinal organoids with TNFα for 24 h increases Bcl-2 and B2M expression. The latter serving as a positive control for NF-kB activation. Error bars represent the s.e.m. (n=2 independent experiments), *P<0.05, **P<0.01, Student’s t-test. (e) Treatment of intestinal organoids with the NF-κB inhibitor JSH-23 for 24 h decreases Bcl-2 and B2M expression. Error bars represent the s.e.m. (n=4 independent experiments), **P<0.01, ****P<0.0001, Student’s t-test. (f) ChIP assay with a p65 antibody on DNA isolated from untreated and TNFα treated organoids. PCR showing enrichment for the Bcl-2 promoter region in the TNFα treated group. (g) Quantitative PCR analysis on DNA derived from the ChIP assay and using the same primer set to detect the Bcl-2 promoter region as shown in f. Error bars indicate the s.e.m. (n=2 independent ChIP assays), *P<0.05, Student’s t-test.
BCL-2 marks crypt base columnar stem cells in the intestine
Previously immunohistochemical analyses revealed that BCL-2 is mostly expressed within the base of both human and murine intestinal crypts17,18. Indeed we found that high BCL-2 expressing cells correlates to the Lgr5-green fluorescent protein (GFP)+ crypt base columnar cell population (Fig. 2a,b). Fluorescence-activated cell sorting (FACS) of various Lgr5-GFP expressing populations (negative, low and high) confirms this observation and reveals that Lgr5-GFPneg. and Lgr5-GFPlow cells, representing differentiated and transit-amplifying cells respectively, display comparable low levels of Bcl-2 expression (Fig. 2c). High Bcl-2 transcript levels are detected only in the Lgr5-GFPhigh ISCs. These results were supported by immunohistochemistry for BCL-2 on human intestinal tissue (Fig. 2d,e). In the distal small intestine (ileum) BCL-2 expression was mostly restricted to the crypt base columnar cells (Fig. 2d). Higher expression was also seen in the crypt base of human colon albeit less restricted to a specific population in line with the more indistinct nature of Lgr5+ ISCs in this tissue (Fig. 2e).
(a) Immunohistochemistry for GFP (left) and BCL-2 (right) stained sections of the small intestine of Lgr5-GFP mice. BCL-2 staining shows a similar expression distribution as the Lgr5-GFP+ cells within crypts. Scale bars, 50 μm. (b) Graph depicting the percentage of cells at particular cell positions within crypts that are positive for Lgr5-GFP and BCL-2. A similar distribution of crypt base specific expression can be found in both cases. Error bars represent s.e.m. (n=3 mice). (c) FACS graph indicating the sorting gates for Lgr5-GFPnegative, Lgr5-GFPlow and Lgr5-GFPhigh cell populations (left) and bar graph depicting the relative messenger RNA (mRNA) expression of Bcl-2 within the indicated populations showing the highest expression in the Lgr5-GFPhigh population (right panel). Error bars represent the s.e.m. (n=3 mice), *P<0.05, Student’s t-test. (d) BCL-2 staining of human ileum tissue. Scale bar, 50 μm. (e) BCL-2 staining of human colonic tissue. Scale bar, 50 μm.
Bcl-2 is dispensable for intestinal homeostasis
To study the functional relevance of Bcl-2 in the intestine we generated Bcl-2 knockout mice by crossing a previously described Cre-inducible Bcl-2 knockout strain (Bcl-2fl/fl)19, which allows for inactivation of the gene by removing the second exon, with a germ-line Cre deleter strain (PGK-Cre; Supplementary Fig. 2a,b). These Bcl-2−/− mice displayed characteristic features that have been reported before in the context of Bcl-2 knockout animals: they are of reduced size compared with both Bcl-2+/+ and Bcl-2+/− littermates and display an accelerated greying of the fur (Supplementary Fig. 3a)20,21,22. Following confirmation of the lack of Bcl-2 expression both in the intestinal epithelial cell and the lymphocytic cell compartment (Supplementary Fig. 3b), we evaluated intestinal tissue morphology and differentiation patterns (Supplementary Fig. 3c–f). Alcian blue, lysozyme and villin staining did not reveal alterations in the sizes or distributions of the goblet cell, Paneth cell or total enterocyte populations, respectively (Supplementary Fig. 3d). Furthermore, Bcl-2 loss bears no impact on the amount or distribution of proliferating cells in the intestine (Supplementary Fig. 3e) and a small apparent reduction in the number of apoptotic cells was not found to be significant (Supplementary Fig. 3f). These results show that no cellular and intestinal tissue phenotype could be detected in Bcl-2 deficient mice, and therefore suggest that this gene is dispensable for normal intestinal homeostasis. To what degree the reduced body size and weight is due to impaired intestinal function remains unclear but many other phenomena, besides nutrient malabsorption, could underlie this observation.
Bcl-2 is dispensable for regeneration following radiation
Recently it was reported that Lgr5+ cells are required for intestinal regeneration following irradiation-induced damage23,24. To investigate if Bcl-2 deficient Lgr5+ cells remain capable of epithelial regeneration in this setting, we crossed Bcl-2fl/fl mice with Lgr5-CreER mice to generate Lgr5-CreER.Bcl-2fl/fl mice in which the Bcl-2 gene can be deleted following tamoxifen injection. Lgr5-CreER mice display a marked mosaicism for Lgr5 promoter-driven EGFP-IRES-CreER expression, which allowed us to directly compare Lgr5-GFP+.Bcl-2−/− crypts and Lgr5-GFP−.Bcl-2+/+ crypts. Efficient inducible deletion of Bcl-2 was observed after tamoxifen induction by immunohistochemistry and PCR analysis and >90% of Lgr5-GFP+ crypts displayed Bcl-2 locus recombination and had lost BCL-2 expression (Supplementary Fig. 4a–c). Of note, Bcl-2 loss in the ISC compartment does not alter the proportion of Lgr5-expressing cells indicating that Bcl-2 is non-essential to the crypt base columnar ISC compartment in homeostasis (Supplementary Fig. 4d). In addition, the Lgr5-CreER.Bcl-2fl/fl model permitted us to confirm that also epithelial specific loss of Bcl-2 does not impact on intestinal morphology, the abundance of differentiated cell types (Supplementary Fig. 5a–g) or the number of proliferating and apoptotic cells (Supplementary Fig. 6a–d).
In line with previous results assessing the role of Bcl-2 in radiation resistance25,26, we observed that loss of Bcl-2 results in increased apoptosis following a dose of 6 Gy irradiation as evidenced by cleaved caspase-3 staining at early time points (Fig. 3a,b). Although a decreased proportion of Lgr5-GFP+ cells can be detected early post-irradiation, 3 weeks after irradiation the number of Lgr5+ ISCs has restored to the untreated situation (Fig. 3c) and a similar trend can be observed for the number of Lgr5+ crypts (Supplementary Fig. 7a). Critically, we confirmed that the Lgr5-GFP+ cells that regenerated the intestinal tissue are Bcl-2 deficient, ruling out the possibility that the regenerative potential resides in rare Lgr5+ cells that escaped Bcl-2 loss (Supplementary Fig. 7b). Combined these results indicate that, following irradiation-induced DNA damage at this dosing regimen, Bcl-2 protects against the occurrence of apoptosis in the crypt compartment but at the same time is dispensable for intestinal tissue regeneration.
(a) Panel of sections stained for cleaved caspase-3 (CC-3) in Lgr5.Bcl-2+/+ mice (top) and Lgr5.Bcl-2fl/fl mice (bottom) 5 weeks after tamoxifen induction and 4 h after 6 Gy full-body irradiation. Scale bars, 100 μm. (b) Graph displaying the percentage of CC-3 foci per crypt for Lgr5.Bcl-2+/+ and Lgr5.Bcl-2fl/fl mice at the indicated time points post 6 Gy irradiation. A minimum of 80 crypts was counted for each genotype and time point. Error bars represent the s.e.m., *P<0.05, ****P<0.0001, Student’s t-test. (c) Graph showing the percentage of Lgr5-GFP+ cells in the small intestine for non-irradiated controls and post-irradiated mice at the indicated days (n=3 mice per condition, per time point). Analyses are performed by FACS. Error bars represent the s.e.m., NS, not significant, *P<0.05, Student’s t-test.
Bcl-2 facilitates tumourigenesis in ISCs
We identified Bcl-2 as a putative shared signal between ISCs and differentiated cells harbouring active NF-κB signalling, and speculate that this molecule might be involved in facilitating transformation in both cell types. To test this hypothesis we employed mice that allow for inducible inactivation of the tumour suppressor gene Apc (Apcfl/fl). Apc is a negative regulator of the Wnt pathway and loss of APC function results in intestinal hyperproliferation and adenoma formation27. Inactivating mutations in the homologous human APC gene, initiate a sizeable proportion of human CRCs28.
Here we crossed Lgr5-CreER.Bcl-2fl/fl mice with Apcfl/fl mice (Lgr5.Bcl-2fl/fl.Apcfl/fl) to determine the relevance of Bcl-2 for intestinal tumour initiation. Critically, we confirmed all mice were highly congenic towards the C57BL/6 background by a single-nucleotide polymorphism (SNP) assay (Supplementary Fig. 8a,b). We observed that 30 days after tamoxifen administration, simultaneous inactivation of Apc and Bcl-2 resulted in significantly impaired adenoma formation (Fig. 4a,b). Furthermore, even heterozygous loss of Bcl-2 resulted in a threefold reduction in the number of adenomas (Fig. 4b). Bcl-2 loss was associated with reduced adenoma sizes (Fig. 4c). In a separate experiment we studied the rate at which adenomas developed in the presence or absence of functional Bcl-2. We detected a significant delay in the onset of intestinal adenoma associated symptoms in mice that had impaired Bcl-2 function within adenomatous clones (Fig. 4d). Mice that had to be killed in the Lgr5.Bcl-2fl/fl.Apcfl/fl group had many fewer adenomas but suffered invariably from a few large tumours in the caecum that prompted killing of the mice (Fig. 4e,f). Importantly, we noticed a much higher number of cystic lesions in the intestines of Lgr5.Bcl-2fl/fl.Apcfl/fl mice following induction (Fig. 4g). These lesions have previously been described as the consequence of inactivating Apc only in differentiated villus cells and contrast with the large, multiglandular adenomas that arise from ISCs5. Together these observations strongly suggest that Bcl-2 is a critical gene required for stem cell initiated intestinal tumorigenesis.
(a) Representative hematoxylin and eosin (H&E) stained sections of two small intestines of the Lgr5.Bcl-2+/+.Apcfl/fl and Lgr5.Bcl-2fl/fl.Apcfl/fl mice at day 30 post induction. Arrows indicate an adenomatous lesion. Scale bars, 2 mm. (b) Graph showing the number of adenomas at day 30 post induction for Lgr5.Bcl-2+/+.Apcfl/fl, Lgr5.Bcl-2+/fl.Apcfl/fl and Lgr5.Bcl-2fl/fl.Apcfl/fl mice. Each dot represents a mouse (n=5), error bars represent s.e.m. **P<0.01, ****P<0.0001, Student’s t-test. (c) Graph showing the adenoma size at day 30 post induction in Lgr5.Bcl-2+/+.Apcfl/fl, Lgr5.Bcl-2+/fl.Apcfl/fl and Lgr5.Bcl-2fl/fl.Apcfl/fl mice. Each dot represents a measurement of the adenoma size (minimal n=32 per genotype), error bars represent the s.e.m. **P<0.01, ***P<0.001, Student’s t-test. (d) Kaplan–Meier plot demonstrating the surviving fraction at the indicated time points post induction of Lgr5.Bcl-2+/+.Apcfl/fl, Lgr5.Bcl-2+/fl.Apcfl/fl and Lgr5.Bcl-2fl/fl.Apcfl/fl mice (minimal n=10 mice per genotype). P value by log-rank test. (e) Graph indicating the number of adenomas per mice at the time of culling in the survival analysis (n=4). Error bars represent the s.e.m., ***P<0.001, ****P<0.0001, Student’s t-test. (f) Representative images of distal part of the small intestines of Lgr5.Bcl-2+/+.Apcfl/fl and Lgr5.Bcl-2fl/fl.Apcfl/fl mice, included in the survival analysis, at the time of termination. (g) Representative immunohistochemical panel of Apc-deficient adenomas (top panel) and cyst-like structures (bottom panel) in the small intestines of mice of the indicated genotypes 30 days after induction; H&E (left), Lgr5-GFP (middle-left), β-catenin (middle-right) and BCL-2 (right) staining. Scale bars, 50 μm. The graph displays the fraction of cyst-like structures observed in mice of the indicated genotypes at moment of termination. Each dot represents a mouse (n=3), error bars represent the s.e.m. ****P<0.0001, Student’s t-test.
Inhibition of BCL-2 impairs intestinal tumorigenesis
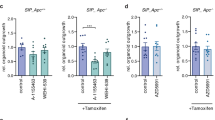
Having identified Bcl-2 as a critical facilitator of tumour formation, we wished to formally confirm that the consequences of impaired Bcl-2 function are specific for Apc deficient cells. Therefore, we employed a clonogenic assay using intestinal organoid cultures derived from untreated Lgr5-CreER.Apcfl/fl and Lgr5-CreER.Apc+/+ mice and, following tamoxifen induction in vitro, treated them with increasing doses of ABT-199, a selective BCL-2 inhibitor29. We observed that BCL-2 inhibition specifically reduced the outgrowth of Apc−/− cells into spheroids, while leaving wild-type cells unaffected (Fig. 5a,b). These results were not recapitulated when using a specific inhibitor (WEHI-539) of another Bcl-2 family member, BCL-XL, confirming that specifically BCL-2 inhibition is responsible for these observations (Fig. 5c). This is also in line with the observations following genetic Bcl-2 ablation in our mouse studies. Next, we established that ABT-199 could prevent adenoma formation in vivo. Specifically, we found that oral application of the BCL-2 inhibitor, once every 2 days, in Lgr5-CreER.Apcfl/fl mice for 27 days after tamoxifen administration resulted in a significant reduction in the average number of adenomas (Fig. 5d,e). We also corroborated our previous observation that transformation in a context of impaired BCL-2 function results in formation of indolent lesions, reminiscent of the cystic structures detected following Apc inactivation in differentiated cells (Fig. 5f).
(a) Representative bright field images of untreated (left) and BCL-2 inhibitor (ABT-199) treated (right) organoid structures obtained from Lgr5.Apc+/+ (top) and Lgr5.Apcfl/fl (bottom) mice 5 days after induction with tamoxifen. Scale bar, 50 μM. (b) Graphs depict the relative outgrowth of structures 7 days after passaging for the indicated genotype and ABT-199 dose at time of induction. Each dot represents a replicate (minimal n=5 per condition), error bars indicate the s.e.m. **P<0.01, ***P<0.001, Student’s t-test. (c) Graph depicting the relative outgrowth of Apc−/− structures 7 days after passaging treated with BCL-XL inhibitor (WEHI-539, 1,000 nM) at time of induction. Each dot represents a replicate (minimal n=5 per condition), error bars indicate the s.e.m. NS, not significant, Student’s t-test. (d) Representative H&E images of sections of the small intestine of Lgr5.Apcfl/fl mice, which were killed at day 27 post induction. The ABT-199 treated cohort received 100 mg kg−1 ABT-199 starting 2 days before induction for three consecutive days followed by administration every other day until day 27. Scale bars, 2 mm. (e) Graph displaying the number of adenomas at day 27 post induction. Each dot represents a mouse (minimal n=4 per condition), error bars represent the s.e.m. *P<0.05, Student’s t-test. (f) Plot showing the quantification of the fraction of cyst-like structures in control and ABT-199 treated mice. Each dot represents a mouse (minimal n=3 per condition), error bars represent the s.e.m. ***P<0.001, Student’s t-test.
BCL-2 inhibition induces cell death in early adenomas
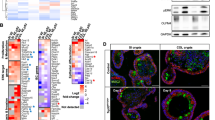
To determine the importance of Bcl-2 in established adenomas we evaluated Bcl-2 expression in adenomatous tissue. First, we found that Bcl-2 messenger RNA is upregulated following Apc-loss by isolating tdTomato+/Apc−/− cells from AhCreER.Apcfl/fl.Lox-STOP-Lox-tdTomato+/− mice 14 days after induction (Fig. 6a). Second, we performed immunohistochemical analysis of adenoma tissue that developed in induced Lgr5-CreER.Apcfl/fl mice, and in a spontaneous model of intestinal tumourigenesis (ApcMin)30. This analysis revealed increased BCL-2 expression in transformed glands compared with normal tissue (Fig. 6b; Supplementary Fig. 9a). We observed that, similar to normal crypts, BCL-2 expression matches Lgr5-GFP expression suggesting a specific role of Bcl-2 in stem-like cells in adenomas. A similar pattern of BCL-2 distribution was seen in human adenomas (Supplementary Fig. 9b). We speculated that BCL-2 inhibition might promote cell death in established adenomas. To test this, we treated Apc-deficient organoids with a BCL-2 inhibitor and found a significant reduction in the percentage of viable structures after 72 h (Fig. 6c; Supplementary Fig. 9c). We corroborated this in vitro observation by treating adenoma bearing Lgr5-CreER.Apcfl/fl mice (3 weeks after induction) with ABT-199 for four consecutive days, which revealed efficient induction of cell death within these lesions (Fig. 6d). Together these results suggest that Apc−/− cells are dependent on Bcl-2 for survival and clonogenic expansion, both during tumour initiation as well as in established adenomatous tissue.
(a) Isolation of Apc+/+ and Apc−/− cells at day 14 post low-level clone induction in AhCreER.Apcfl/fl.Lox-STOP-Lox-tdTomato+/− mice. The left panel represents the FACS sort analysis and the graph shows the relative Bcl-2 expression of the Apc+/+ compared with the Apc−/− cell population. Error bars represent s.d. (n=3 per condition), ***P<0.001, Student’s t-test. (b) Lgr5-GFP and BCL-2 stained adenomas in a Lgr5.Apcfl/fl mouse 5 weeks post induction demonstrating overexpression of Bcl-2 in the Lgr5-GFP+ adenomatous glandular structures. Scale bars, 100 μm. (c) Representative images of Apc-deficient spheroids; upper image showing a viable spheroid of the control group and the lower image demonstrating a non-viable spheroid as observed after ABT-199 treatment. The graph displays the amount of viable spheroids counted at 72 h after the start of ABT-199 treatment for the indicated doses. Scale bars, 50 μM. Dots indicate replicates (minimal n=5 per condition), error bars represent the s.e.m. *P<0.05, ***P<0.001, ****P<0.0001, Student’s t-test. (d) Lgr5.Apcfl/fl mice with established adenomas 4 weeks following induction were treated with ABT-199 for 4 consecutive days. Cell death within these adenomas was compared with cell death within adenomas of untreated Lgr5.Apcfl/fl mice by counting the amount of cleaved caspase-3 (CC-3) positive foci for each glandular structure. Each dot represents a gland (minimal n=62 glands per condition), error bars indicate the s.e.m. ****P<0.0001, Student’s t-test. (e) FACS graphs indicating the sorting gates for AhCreER.Apc+/+ and AhCreER.Apc−/− cells that have lost a JC-1 signal. (f) Bar graph showing the percentage of cells undergoing mitochondrial depolarization for the indicated concentrations of the BID peptide. The percentage of cells undergoing mitochondrial depolarization was measured with JC-1 staining and was calculated relative to the negative (DMSO) and positive (CCCP) control.
Apc deficiency primes intestinal cells for apoptosis
To further investigate if Apc depletion causes cellular stress that leads to increased apoptotic priming, we adopted a previously described mitochondrial priming assay31. This assay assesses the readiness of cells to undergo apoptosis when treating cells with increasing concentrations of pro-apoptotic peptides. AhCreER.Apcfl/fl organoid cultures allowed us to determine the sensitivity of Apc depleted cells to undergo apoptosis. After induction, control and Apc deficient cells were treated with different concentrations of a synthetic BH3-interacting domain death agonist (BID) peptide, which is a pro-apoptotic protein that binds anti-apoptotic Bcl-2 family members, including BCL-2, and causes a shift in the balance towards apoptosis induction. Next, the percentage of cells undergoing mitochondrial depolarization, a critical event in the apoptotic cascade, was measured by flow cytometry (Fig. 6e). Our results indicate that a significantly higher fraction of Apc deficient cells undergo BID-induced apoptosis compared with control cells at increasing BID-peptide concentrations (Fig. 6f). Thus, Apc inactivation causes cellular stress that primes intestinal epithelial cells for apoptosis. However, Bcl-2 expression alleviates this effect and contributes to efficient transformation of ISCs.
Bcl-2 allows transformation of differentiated cells
Our results so far indicate that Bcl-2 is an NF-κB target in intestinal epithelial cells and required for transformation of ISCs. Previously it was shown that differentiated epithelial cells with activated NF-κB signalling can effectively initiate adenoma formation7. Hence we hypothesized that expression of Bcl-2 is sufficient for differentiated cells to withstand Apc-loss-induced apoptosis and generate clonogenic cells. To test this, we transduced AhCreER.Apcfl/fl organoid cultures with a Bcl-2-red fluorescent protein (RFP)-expressing vector (Bcl-2(OE)-RFP) or with an RFP-expressing control vector. We then generated single-cell clones and identified a line that showed physiological levels of Bcl-2 (Fig. 7a). Overexpressing Bcl-2 specifically enhanced the organoid forming capacity of cells that underwent Apc-loss, while had no effect on wild-type cells (Fig. 7b), suggesting that Bcl-2 increases the population that can be successfully transformed. Subsequently, we stained the AhCreER.Apcfl/fl cultures for CD44 to separate the ISC and transit-amplifying cells (CD44high) from the more differentiated cells (CD44low; Fig. 7c). CD44high cells efficiently generated new organoid cultures while CD44low cells failed to do so, confirming the functional identity of both populations (Fig. 7c). Next, we tested the clonogenic outgrowth of the two populations (CD44low and CD44high) both with and without Bcl-2 overexpression following inactivation of Apc (Fig. 7d). In all cases Bcl-2 overexpression increased the proportion of clonogenic cells likely reflecting that it prevents apoptosis caused by cellular stress associated with FACS sorting and single-cell plating. However, most importantly, Bcl-2 overexpression greatly enhanced the capacity for clonogenic outgrowth of differentiated CD44low cells that underwent Apc inactivation (Fig. 7d). This result suggests that Bcl-2 overexpression can support transformation in differentiated cells not normally capable of clonogenic outgrowth.
(a) Images displaying single-cell sorted RFP+ clones for the RFP-only transduced and Bcl-2(OE).RFP organoids (left panel). Bar graph showing gene expression analysis which indicates a eightfold increase in Bcl-2 expression of the Bcl-2(OE) organoids compared with the wild type (WT) control. Error bars indicate s.e.m. (n=3 technical replicates per group). Scale bar, 100 μM. (b) Graphs displaying the relative outgrowth of WT-RFP and Bcl-2(OE)-RFP organoids after passaging in a control setting and with Apc inactivation. Each dot represents a replicate (minimal n=4 per condition), error bars indicate the s.e.m. NS, not significant, *P<0.05, Student’s t-test. (c) FACS graph showing the sorting gates for CD44low and CD44high cells (left panel). Bar graph showing the clonogenic outgrowth of the CD44high cells in comparison to the CD44low cells (right panel). Error bars indicate the s.e.m. (minimal n=5 per condition), ****P<0.0001, Student’s t-test. (d) Graph on the left displaying the outgrowth percentages of the CD44low expressing cells and on the right of the CD44high expressing cells; in both graphs the outgrowth percentage is shown for the WT, WT+Bcl-2(OE), Apc−/− and Apc−/−+Bcl-2(OE) groups. For all conditions cells have been single-cell sorted followed by tamoxifen induction. Dots indicate replicates (minimal n=5 per condition), error bars represent the s.e.m. NS, not significant, *P<0.05, **P<0.01, ***P<0.001, Student’s t-test.
Discussion
Previous studies on constitutive Bcl-2 knockout mouse models demonstrated discrepancies regarding the effects of Bcl-2 deficiency on the intestinal phenotype20,21,22. One study observed distorted villi throughout the small intestine and impaired proliferation20, whereas in two other models no intestinal abnormalities were detected21,22. Here we also show that Bcl-2 is dispensable for intestinal homeostasis and no morphological impact of Bcl-2 loss could be detected. In contrast, Bcl-2 protects ISCs against irradiation-induced apoptosis, although Bcl-2 deficient cells remain competent to contribute to intestinal regeneration. Our findings reveal that the NF-κB target gene Bcl-2 is an important facilitator of oncogenic transformation within the intestine. A model summary of our findings is presented in Fig. 8. We demonstrate that the loss of Bcl-2 reduces outgrowth of oncogenic Apc-deficient clones. Critically the effects of Bcl-2 loss are specific for (pre)malignant lineages. Hence our results uncover a synthetic lethal interaction between Bcl-2 and Apc32. Correspondingly, we have shown that Apc-loss is associated with an increased intracellular stress leading to apoptotic priming that is normally being evaded by BCL-2 function and by Bcl-2 upregulation in Apc−/− adenomatous tissue. This is in line with previous investigations on the role of anti-apoptotic Bcl-2 family members as synthetic lethal partners in the development of haematological malignancies. Interestingly, there appears to be a clear lineage specificity regarding which Bcl-2 family members are required for malignant transformation of specific cell types. For example, Bcl-xL is required for lymphoma development in a myc-driven mouse model while Bcl-2 is dispensable in this setting33,34. In contrast, for thymic lymphoma development in a p53-deficient mouse model Bcl-xL is superfluous and Mcl-1 is essential35. Similarly, our own results indicate that also the stage of disease is related to which family members are most essential in blocking apoptosis36. In contrast to the early disease studied here, in established human CRCs BCL-XL seems more critical36. It will be interesting to investigate at what exact stage, or with what genetic aberration this change in dependency corresponds.
(a,b) Apc-loss causes cellular stress which primes the Bcl-2 deficient differentiated cells to undergo apoptosis whereas ISCs are rescued by high Bcl-2 expression. As a result, only the ISC population is able to undergo malignant transformation upon Apc inactivation. However, when differentiated cells gain Bcl-2 expression, these cells are also able to efficiently transform (b). This suggests that Bcl-2 expression is an important prerequisite for intestinal epithelial cells to transform. (c) The survival of Apc depleted ISCs can be impaired by treatment with BCL-2 inhibitors and therefore we propose that BCL-2 could serve as an effective chemopreventive target for CRC development.
Strikingly, our data revealed an increased formation of cyst-like structures on combined Apc and Bcl-2 loss within the Lgr5+ ISCs at the expense of adenoma formation. Morphologically identical cystic structures are a consequence of Apc-loss within the differentiated cell compartment5. Furthermore, the formation of similar structures was reported on simultaneous Lgr5-CreER-mediated intestinal inactivation of Apc and Rac1, an upstream activator of NF-κB (ref. 14). We assume these lesions do, in principle, also occur in models in which Apc-loss is induced in the whole-epithelial population, but in these cases the cysts are usually overgrown by the adenomatous expansions that occur from transformation of the stem cell pool. This suggests that these structures do not reflect a gained property of Bcl-2 deficient cells, rather they are more visible given the lack of efficient adenoma formation. More dedicated studies are required to strengthen this amenable concept.
Overall these observations lead us to speculate that Bcl-2 expression might be the critical mediator allowing NF-κB-activated intestinal epithelial cells to efficiently transform. This remains to be formally demonstrated in vivo, although we have already shown that Bcl-2 expression is sufficient to render differentiated cells in organoid cultures amendable to transformation as they give rise to clonogenic cells.
Because BCL-2 inhibition impedes the process of adenoma formation, pharmacological inhibition could play a role as preventive strategy against the development of colon carcinomas in populations that are at high risk for developing this disease. In particular patients who are diagnosed with a genetically inherited disorder, like familial adenomatous polyposis or hereditary non-polyposis CRC (known as Lynch syndrome), have an enhanced lifetime risk of developing CRC and would greatly benefit from effective chemoprevention37. The need for such interventions is emphasized by the fact that current chemoprevention regimes, for example, daily intake of non-steroidal anti-inflammatory drugs, have not proven to be sufficient in circumventing the need for other strategies, like two yearly colonoscopies and colectomy in early adulthood, to avert malignant disease38. It is encouraging to note that the BCL-2 inhibitors have already been used successfully for other neoplastic diseases and are continuously improving with respect to the reduced side-effects29,39.
Methods
Animal experiments
Lgr5-EGFP-IRES-CreER, PGK-Cre, AhCreER, Bcl-2fl/fl, Apcfl/fl and Lox-STOP-Lox-(LSL)-tdTomato mice all have been described previously19,27,30,40,41,42,43. All mice were bred and housed according to UK Home Office Guidelines. The genetic backgrounds of mice were assessed at the DartMouse Speed Congenic Core Facility at the Geisel School of Medicine at Dartmouth. DartMouse uses the Illumina, Inc. (San Diego, CA) GoldenGate Genotyping Assay to interrogate 1449 SNPs spread throughout the genome. The raw SNP data were analysed using DartMouse’s SNaP-Map and Map-Synth software, allowing the determination for each mouse of the genetic background at each SNP location. This analysis confirmed that all mice involved in the survival analysis were highly congenic towards the C57BL/6 background (Fig. 4d, and Supplementary Fig. 8). Tamoxifen was dissolved in sunflower oil and administered via intraperitoneal (i.p.) injections. Lgr5-EGFP-IRES-CreER. Bcl-2fl/fl mice were induced with in total 6 mg tamoxifen i.p. on three consecutive days (2 mg per day). Lgr5-EGFP-IRES-CreER.Apcfl/fl.Bcl-2fl/fl mice were respectively induced with 4 and 2 mg tamoxifen i.p. for two consecutive days. AhCreER.LSL-tdTomato+/− mice are i.p. induced with 40 mg kg−1 β-naphthoflavone and 2 mg tamoxifen. Mice received 6 Gy total body irradiation (2.5 Gy per min). For the survival analysis mice were terminated when showing symptoms of anaemia in combination with weight loss and/or other signs of physical discomfort. At time of inclusion both male and female mice between the ages of 6 and 12 weeks were used. All animals were cared for at the Cancer Research UK Cambridge Institute Biological Resources Unit according to Home Office regulations.
Human materials
All biological materials were obtained with informed consent from all subjects according to the Medical Research Involving Human Subjects Act (WMO) following approval of the ethical medical committee of the Academic Medical Center Amsterdam.
Drug administration
Mice were treated with the ABT-199 compound (purchased from Active Biochemicals) or vehicle-only at a dose of 100 mg kg−1 daily for each protocol. ABT-199 was dissolved in 60% phosal 50PG, 30% polyethylene glycol 400 and 10% ethanol and administered by oral gavage. Treatment started 2 days before tamoxifen induction. Short-term experiments involved treatment for 4 days and the maximum treatment duration for long-term experiments was 27 days with ABT-199 administration on every other day.
Immunohistochemistry
Dissected intestines are prepared as swiss rolls and fixed in a 10% formalin solution for 24 h before paraffin embedding. Sections (3 μm) are stained for haematoxylin/eosin and alcian blue. Immunohistochemistry was performed according to standard procedures. Antibodies were used as follows: anti-murine BCL-2 1:100 (BD Pharmingen, 554218), anti-human BCL-2 1:100 (DAKO, clone 124), anti-cleaved caspase-3 1:100 (R&D Systems, AF835), anti-GFP (Abcam, ab13970), anti-KI67 1:1000 (Dako), anti-Lysozyme 1:500 (Dako, A0099 M7249), anti-RELA/P65 1:100 (Santa Cruz, sc-372) and anti-Villin 1:100 (Santa Cruz, sc-7672). Cystic lesions were defined as single, distended glandular structures of which the luminal side was covered with an epithelial monolayer.
Crypt culture
Intestinal crypts were derived from the Lgr5-CreER and Lgr5-CreER.Apcfl/fl mice as indicated. Crypts were isolated and cultured from the small intestine as described previously44. In short, isolated intestines were opened longitudinally and villi were gently scraped off. The intestine was washed with PBS and cut into pieces of <0.5 × 0.5 cm. These intestinal tissue pieces were then incubated in 2 mM EDTA at 4 °C for 30 min. After removal of the EDTA solution, the crypts were collected in ice-cold PBS by vigorously shaking the intestinal pieces in a tube and passing them through a 70-μM cell strainer (BD Bioscience). Isolated crypts were seeded in matrigel in a pre-heated 24-well flat-bottom plate. Advanced DMEM/F12 medium (Invitrogen) was used containing the following growth factors: mouse EGF 50 ng ml−1 (Tebu-bio), R-spondin (conditioned medium) and Noggin (conditioned medium)45. R-spondin were produced by stably transfected 293T cells with a plasmid encoding mRSPO1-Fc provided by dr Calvin Kuo at Stanford University, La Jolla, CA. To allow in vitro recombination, (Z)-4-hydroxytamoxifen (Sigma) was added to the medium with a final concentration of 100 nM. Organoids derived from the AhCreER.Apcfl/fl strain were induced with 5 μM (Z)-4-hydroxytamoxifen and 10 μg ml−1 β-naphthoflavone. NF-κB was inhibited in organoid cultures using JSH-23 (40 μM) obtained from Sigma-Aldrich (J4455). NF-κB was activated using TNFα (25 ng ml−1) obtained from eBioscience (14-8321-62). Organoid cultures were transduced with pHEFTIR (control) or pHEFTIR-BCL2 (BCL-2) lentiviral constructs. The constructs were generated as described by Colak et al.36. Following lentiviral transduction, single-cell derived WT-RFP and Bcl-2(OE).RFP organoid cultures were established for further experiments.
Clonogenic assay
Organoid cultures from Lgr5-CreER.Apcfl/fl and Lgr5-CreER.Apc+/+ mice were plated at a density of 150 crypt structures per well. After 3 days organoids were simultaneously induced with (Z)-4-hydroxytamoxifen (100 nM) and treated with ABT-199 or WEHI-539 (MedChem Express, HY-15607) for 72 h. To determine clonogenicity each well was passaged and organoid outgrowth was quantified after 7 days by microscope.
Flow cytometry
The BD FACSAria III and BD Influx cell sorter were used for flow cytometry analysis and cell sorting. The epithelial fraction is identified with an Alexa 546 labeled EpCAM antibody (Biolegend Cat. no. 118212) at a concentration of 0.5 μg ml−1. CD44 low and high fractions were identified with anti-mouse CD44 labelled with fluorescein isothiocyanate (eBioscience). Dead cells were excluded with 4,6-diamidino-2-phenylindole or propidium iodide staining.
Chromatin immunoprecipitation analysis (ChIP)
ChIP assay was performed with a p65 antibody 1:100 (Cell Signaling, #8242). The SimpleChIP Enzymatic Chromatin IP Kit with magnetic beads (Cell Signaling) was used according to the manufacturer’s protocol. Briefly, TNFα treated (25 ng ml−1) and untreated organoids were fixed with 1% formaldehyde for 10 min at room temperature. Glycine was added to quench cross-linking. After washing with ice-cold PBS, cells were lysed and digested with 5 μl micrococcal nuclease (2,000 gel units per μl) per sample for 20 min at 37 °C. Additionally, cells were sonicated to break the nuclear membrane. Lysates were incubated with a p65 antibody and Normal Rabbit IgG overnight at 4 °C. Protein G magnetic beads were added and samples were incubated for 2 h at 4 °C. Protein G magnetic bead pellets were washed with low- and high-salt washes. Next the chromatin was eluted from the antibody/Protein G beads and DNA was eluted with DNA elution buffer. The forward primer 5′-ACACTTTATGTGTGGTTAGAAAGGG-3′ and reverse primer 5′-ACCTGGATCTTTTCTAACAGGATG-3′ were designed to identify the Bcl-2 promoter region and used for PCR and quantitative real-time PCR analysis. PCR analysis was performed with Platinum Taq DNA Polymerase (Invitrogen) and Quantitative real-time PCR was performed with Sybr green (SensiFAST) according to manufacturer’s protocol.
Apoptotic priming assay
Mitochondrial priming was measured as described by Ryan et al.31 AhCreER.Apcfl/fl organoid cultures have been treated with 5 μM (Z)-4-hydroxytamoxifen 4 days before profiling to generate a high percentage of Apc deficient cells. At day four both control and Apc-deficient crypt cultures were collected and trypsinized. Cells were washed in T-EB (300 mM Trehalose, 10 mM HEPES-KOH pH 7.7, 80 mM KCl, 1 mM EGTA, 1 mM EDTA, 0.1% BSA and 5 mM succinate) and then resuspended in T-EB at a concentration of 500,000 cells per ml. The cell solution (in total 100 μl) was then mixed at a 1:1 ratio with T-EB containing 20 μg ml−1 digitonin, 20 μg ml−1 oligomycin and various concentrations of the BID peptide (EDIIRNIARHLAQVGDSMDR; Eurogentec, Maastricht, The Netherlands) and incubated at room temperature for 90 min. Next, JC-1 staining (1 μM) was performed for 30 min at room temperature and mitochondrial depolarization was measured on the FACS Canto (BD Biosciences).
Polymerase chain reactions
Primers have been designed to identify recombination events of exon 2. The forward primer 5′-GAGCTGGATTTGCAGGCAGTTATTT-3′ and reverse primer 5′–TATGATTAAGGGCATTTTCCCACCA-3′ flank exon 2 of the Bcl-2 locus. Genomic DNA was extracted from tissue or sorted cell populations and lysed with Cell Lysis Solution (5 PRIME) with 0.4 mg kg−1 proteinase K for 3 h at 50 °C. Protein precipitation solution (5 PRIME) was used according to manufacturer’s instructions.
RNA isolation and quantitative real-time PCR
RNA is extracted with the Qiagen RNeasy Plus Micro Kit (cat no. 74034). Synthesis of complementary DNA is performed with the Roche Transcriptor High Fidelity cDNA Synthesis kit (cat no. 05091284001). Bcl-2 (cat no. 4331182, RefSeq NM_009741.4) and Rpl19 (cat no. 4331182, RefSeq NM_000981.3) Taqman probes have been used for the Sybr green quantitative PCR with reverse transcription which was performed under standard conditions. The ΔΔCt method has been applied for calculations of gene expression.
Analysis of gene expression and GSEA
Publicly available data sets were downloaded and normalized using robust multi-array average46. GSEA was performed as previously described using the GSEA desktop application (V2.0.14) from the Broad Institute (http://www.broadinstitute.org/gsea/index.jsp)47. The NF-kB target gene signature was derived from Compagno et al.48 and includes 120 genes of which we manually identified the murine homologues. For the drug targets the gene ontology ‘drug target’ category was used. Identification of differentially expressed genes was performed using a t-test and a threshold of P<0.01 was used to determine significance.
Additional information
How to cite this article: van der Heijden, M. et al. Bcl-2 is a critical mediator of intestinal transformation. Nat. Commun. 7:10916 doi: 10.1038/ncomms10916 (2016).
References
Brenner, H., Kloor, M. & Pox, C. P. Colorectal cancer. Lancet 383, 1490–1502 (2014).
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Vermeulen, L. et al. Defining stem cell dynamics in models of intestinal tumor initiation. Science 342, 995–998 (2013).
Vermeulen, L. & Snippert, H. J. Stem cell dynamics in homeostasis and cancer of the intestine. Nat. Rev. Cancer 14, 468–480 (2014).
Barker, N. et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009).
Sangiorgi, E. & Capecchi, M. R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 40, 915–920 (2008).
Schwitalla, S. et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152, 25–38 (2013).
Westphalen, C. B. et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J. Clin. Invest. 124, 1283–1295 (2014).
Jess, T., Rungoe, C. & Peyrin-Biroulet, L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 10, 639–645 (2012).
Perkins, S., Clarke, A. R., Steward, W. & Gescher, A. Age-related difference in susceptibility of Apc(Min/+) mice towards the chemopreventive efficacy of dietary aspirin and curcumin. Br. J. Cancer 88, 1480–1483 (2003).
Rothwell, P. M. et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377, 31–41 (2011).
Arber, N. et al. Celecoxib for the prevention of colorectal adenomatous polyps. N. Engl. J. Med. 355, 885–895 (2006).
Greten, F. R. et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Myant, K. B. et al. ROS production and NF-kappaB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell 12, 761–773 (2013).
Merlos-Suarez, A. et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 8, 511–524 (2011).
Munoz, J. et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent '+4' cell markers. EMBO J 31, 3079–3091 (2012).
Bosari, S. et al. bcl-2 oncoprotein in colorectal hyperplastic polyps, adenomas, and adenocarcinomas. Hum. Pathol. 26, 534–540 (1995).
Merritt, A. J. et al. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J. Cell Sci. 108, 2261–2271 (1995).
Thorp, E. et al. Brief report: increased apoptosis in advanced atherosclerotic lesions of Apoe-/- mice lacking macrophage Bcl-2. Arterioscler. Thromb. Vasc. Biol. 29, 169–172 (2009).
Kamada, S. et al. bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res. 55, 354–359 (1995).
Nakayama, K. et al. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc. Natl Acad. Sci. USA 91, 3700–3704 (1994).
Veis, D. J., Sorenson, C. M., Shutter, J. R. & Korsmeyer, S. J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75, 229–240 (1993).
Buczacki, S. J. et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69 (2013).
Metcalfe, C., Kljavin, N. M., Ybarra, R. & de Sauvage, F. J. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 14, 149–159 (2014).
Sotiropoulou, P. A. et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat. Cell Biol. 12, 572–582 (2010).
Pritchard, D. M., Potten, C. S., Korsmeyer, S. J., Roberts, S. & Hickman, J. A. Damage-induced apoptosis in intestinal epithelia from bcl-2-null and bax-null mice: investigations of the mechanistic determinants of epithelial apoptosis in vivo. Oncogene 18, 7287–7293 (1999).
Shibata, H. et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 278, 120–123 (1997).
Fearon, E. R. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. 6, 479–507 (2011).
Souers, A. J. et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202–208 (2013).
Moser, A. R. et al. ApcMin: a mouse model for intestinal and mammary tumorigenesis. Eur. J. Cancer 31A, 1061–1064 (1995).
Ryan, J. A., Brunelle, J. K. & Letai, A. Heightened mitochondrial priming is the basis for apoptotic hypersensitivity of CD4+ CD8+ thymocytes. Proc. Natl Acad. Sci. USA 107, 12895–12900 (2010).
Brummelkamp, T. R. & Bernards, R. New tools for functional mammalian cancer genetics. Nat. Rev. Cancer 3, 781–789 (2003).
Kelly, P. N., Grabow, S., Delbridge, A. R., Strasser, A. & Adams, J. M. Endogenous Bcl-xL is essential for Myc-driven lymphomagenesis in mice. Blood 118, 6380–6386 (2011).
Kelly, P. N., Puthalakath, H., Adams, J. M. & Strasser, A. Endogenous bcl-2 is not required for the development of Emu-myc-induced B-cell lymphoma. Blood 109, 4907–4913 (2007).
Grabow, S., Delbridge, A. R., Valente, L. J. & Strasser, A. MCL-1 but not BCL-XL is critical for the development and sustained expansion of thymic lymphoma in p53-deficient mice. Blood 124, 3939–3946 (2014).
Colak, S. et al. Decreased mitochondrial priming determines chemoresistance of colon cancer stem cells. Cell Death Differ. 21, 1170–1177 (2014).
Lynch, H. T. & de la Chapelle, A. Hereditary colorectal cancer. N. Engl. J. Med. 348, 919–932 (2003).
Steinbach, G. et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 342, 1946–1952 (2000).
Roberts, A. W. et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374, 311–322 (2015).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Kemp, R. et al. Elimination of background recombination: somatic induction of Cre by combined transcriptional regulation and hormone binding affinity. Nucleic Acids Res. 32, e92 (2004).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Lallemand, Y., Luria, V., Haffner-Krausz, R. & Lonai, P. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 7, 105–112 (1998).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762–1772 (2011).
Heijmans, J. et al. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep. 3, 1128–1139 (2013).
Irizarry, R. A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 4, 249–264 (2003).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Compagno, M. et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature 459, 717–721 (2009).
Acknowledgements
We thank the animal caretakers and other core facilities in CRUK Cambridge Institute that contributed to this study. This work is supported by Cancer Research UK, the Academic Medical Center (Amsterdam), KWF grants (UVA2011–4969, UVA2014–7245, UVA2014–6331 and UVA2014–7587), Worldwide Cancer Research (14–1164), the Maag Lever Darm Stichting (MLDS-CDG 14–03 and MLDS FP13-07) and the European Research Council (ERG-StG 638193).
Author information
Authors and Affiliations
Contributions
All authors contributed to current study including intellectual input, conducting experiments, result analysis, and writing and editing the manuscript. Specifically M.v.d.H., C.D.Z., A.M.N. and S.C. performed experiments. M.v.d.H., C.D.Z., R.K., S.L.M., J.P.M., F.R.G., D.J.W. and L.V. analysed data. M.v.d.H., M.J., D.J.W. and L.V. conceived and designed research. M.v.d.H., D.J.W. and L.V. wrote the draft manuscript. All authors approved the content of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-10 (PDF 6009 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
van der Heijden, M., Zimberlin, C., Nicholson, A. et al. Bcl-2 is a critical mediator of intestinal transformation. Nat Commun 7, 10916 (2016). https://doi.org/10.1038/ncomms10916
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms10916
This article is cited by
-
Epithelial CRL4DCAF2 Is Critical for Maintaining Intestinal Homeostasis Against DSS-Induced Colitis by Regulating the Proliferation and Repair of Intestinal Epithelial Cells
Digestive Diseases and Sciences (2024)
-
ADORA2A promotes proliferation and inhibits apoptosis through PI3K/AKT pathway activation in colorectal carcinoma
Scientific Reports (2023)
-
Molecular mechanisms behind ROS regulation in cancer: A balancing act between augmented tumorigenesis and cell apoptosis
Archives of Toxicology (2023)
-
Expression of apoptosis repressor with caspase recruitment domain (ARC) in familial adenomatous polyposis (FAP) adenomas and its correlation with DNA mismatch repair proteins, p53, Bcl-2, COX-2 and beta-catenin
Cell Communication and Signaling (2021)
-
BCL-XL is crucial for progression through the adenoma-to-carcinoma sequence of colorectal cancer
Cell Death & Differentiation (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.