Abstract
The complement system is important for the host defence against infection as well as for the development of inflammatory diseases. Here we show that C1q/TNF-related protein 6 (CTRP6; gene symbol C1qtnf6) expression is elevated in mouse rheumatoid arthritis (RA) models. C1qtnf6−/− mice are highly susceptible to induced arthritis due to enhanced complement activation, whereas C1qtnf6-transgenic mice are refractory. The Arthus reaction and the development of experimental autoimmune encephalomyelitis are also enhanced in C1qtnf6−/− mice and C1qtnf6−/− embryos are semi-lethal. We find that CTRP6 specifically suppresses the alternative pathway of the complement system by competing with factor B for C3(H2O) binding. Furthermore, treatment of arthritis-induced mice with intra-articular injection of recombinant human CTRP6 cures the arthritis. CTRP6 is expressed in human synoviocytes, and CTRP6 levels are increased in RA patients. These results indicate that CTRP6 is an endogenous complement regulator and could be used for the treatment of complement-mediated diseases.
Similar content being viewed by others
Introduction
The complement system is a component of the innate immune system, and plays important roles in host defence against microbial infection1. The complement system is also suggested to be involved in the pathogenesis of a group of inflammatory diseases. Rheumatoid arthritis (RA) is one of such diseases in which serum concentrations of complement active fragments (C3a and C5a) are elevated2,3. The development of arthritis is suppressed by the deficiency of the complement system in RA models4,5,6,7. The importance of complement activation is also suggested in other inflammatory diseases such as multiple sclerosis (MS) and glomerulonephritis8,9,10.
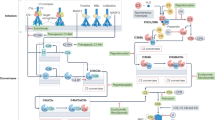
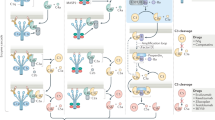
Complement activation is a proteolytic cascade consisting of three distinct pathways11. The classical pathway (CP) is initiated by C1q binding to immune complexes. The lectin pathway (LP) is triggered by binding of mannose-binding lectin or ficolins to pathogen-associated carbohydrate motifs. In the alternative pathway (AP), C3(H2O) is initially produced by spontaneous hydrolysis of the internal thioester bond in C3 (Supplementary Fig. 1). Then, factor B binds C3(H2O), and the complex is activated by factor D to generate fluid-phase C3 convertase (C3(H2O)Bb) (tick-over). On the surface of pathogens, C3(H2O)Bb cleaves C3 into C3a and C3b, resulting in C3b deposition. C3b, factor B and factor P form C3 convertase (C3bBbP), which amplifies the reaction (amplification loop). C3b, in combination with Bb, cleaves C5, generating anaphylatoxin C5a and C5b, which form the membrane attack complex (MAC). C3a and C5a have chemotactic and anaphylactic activities; they recruit inflammatory cells to inflammatory sites and activate them to produce inflammatory cytokines1,11.
RA, an autoimmune disease that attacks multiple joints, affects almost 1% of the world population. Inflammatory cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-6, IL-1 and IL-17 play important roles in RA pathogenesis; antibodies and inhibitors against these cytokines are successfully used to treat the disease12,13. High levels of autoantibodies are a feature of RA, and antibodies against B cells are also effective in treatment of RA14.
To identify novel therapeutic targets for the treatment of RA, we analysed gene expressions in two RA models; human T-cell leukaemia virus type-I transgenic (Tg) mice and IL-1 receptor antagonist (IL-1Ra)-deficient (knockout) mice15,16. We found that the expression of C1qtnf6 gene, which encodes the C1q/TNF-related protein 6 (CTRP6) protein, was highly enhanced in both models17. This gene is also highly expressed in the placenta under physiological conditions18. CTRP6 is a glycoprotein of molecular mass of 29 kDa consisting of four domains, these are signal peptide, short N-terminal variable region, collagen domain and C-terminal C1q domain, and is secreted in the serum-forming oligomeric forms19. Proteins that contain the C1q domain are classified as members of the C1qTNF family, because the crystal structure of the C1q domain resembles both complement component C1q and TNF-α (ref. 20). The C1q domain of human and mouse CTRP6 are highly homologous (82% amino-acid identity, Supplementary Fig. 2a). CTRP family members have been implicated in host defence, inflammation and glucose metabolism19,21.
In this study, by generating C1qtnf6−/− and Tg mice, we demonstrate that CTRP6 is a novel regulator of the complement AP and plays important roles in the maintenance of pregnancy and the pathogenesis of disease models such as collagen-induced arthritis (CIA)22, anti-collagen antibody-induced arthritis (CAIA)6, experimental autoimmune encephalomyelitis (EAE)23 and Arthus reaction24. Because CTRP6 levels are also increased in the serum of RA patients and CTRP6 exerts a potent therapeutic effect on CIA, we suggest that CTRP6 could be used clinically to treat RA and other complement-mediated diseases.
Results
Generation of C1qtnf6−/− mice
Because C1qtnf6 is highly expressed in mouse RA models (Supplementary Fig. 2b), we generated C1qtnf6−/− mice to elucidate the pathological roles of CTRP6 in the development of arthritis (Supplementary Fig. 2c–e). C1qtnf6−/− mice were fertile and showed no obvious abnormalities before 6 months of age. However, the frequency of obtaining homozygotes from the matings of (129P2/Ola × C57BL/6) F1 background mice was less than the expected Mendelian ratio (wild type (WT):heterozygote:homozygote=159 (31.7%):245 (48.9%):97 (19.4%), P=0.021). We used mice after backcrossing with C57BL/6 mice by the high-speed congenic strategy25, in which C57BL/6-specific allotypes were confirmed in 84 microsatellite markers, throughout the experiments. Aged C1qtnf6−/− mice produced significantly higher levels of autoantibodies, such as antibodies to nuclear antigens (ANAs), IgG- and IgM-type rheumatoid factor (RF) (Supplementary Fig. 3a), although cell populations of lymphoid tissues (Supplementary Fig. 3b–d) and antibody production against thymus-dependent and -independent antigens were normal (Supplementary Fig. 3e–h). Renal function was also normal in these C1qtnf6−/− mice (Supplementary Fig. 3i).
C1qtnf6−/− mice are susceptible to CIA
We next examined the development of CIA in C1qtnf6−/− mice. Mice were immunized with chicken type-II collagen (IIC) emulsified in complete Freund’s adjuvant (CFA) containing a low concentration of Mycobacterium tuberculosis (1.65 mg ml−1) to induce mild arthritis. In WT mice under this regimen, the induced arthritis was mild. By contrast, in C1qtnf6−/− mice, the onset of arthritis was earlier, and the severity score was higher than in WT mice, although the incidence of arthritis was similar between the two strains (Fig. 1a). Histology of the joints of WT mice revealed mild pathological changes, whereas that of C1qtnf6−/− joints revealed much more severe changes, including proliferation of synovial lining cells, infiltration of inflammatory cells and bone destruction associated with pannus formation (Fig. 1b). The number and percentage of CD4+ T cells in lymph node (LN) were similar between C1qtnf6−/− and WT mice, whereas the B220+ B-cell population was significantly expanded in C1qtnf6−/− mice (Fig. 1c). Moreover, IIC-specific IgG levels after IIC immunization were significantly higher in C1qtnf6−/− than in WT mice (Fig. 1d). The proliferative response to IIC of LN cells in C1qtnf6−/− mice was similar to that of WT mice, suggesting that T-cell priming was normal (Fig. 1e). These results suggest that C1qtnf6−/− mice are more susceptible to CIA than WT mice.
(a) Incidence (left) and severity score (right) of CIA (WT: n=10, knockout (KO): n=9). *P<0.05. χ2-test and Mann–Whitney U-test. (b) Histopathology (left) and histological score (right) of the ankle joints at day 42 after primary immunization (WT, KO: n=9 each). One of representative histologies is shown. Haematoxylin and eosin staining. Tibia, talus and navicular bone are represented as Tib, Tal and Nav, respectively. Bone destruction by pannus formation into talus and navicular bone associated with the presence of osteoclasts and fibroblasts in the KO joint. Scale bar, 300 μm. ***P<0.001. Student’s t-test. (c) At day 42 after immunization, cell number (left) and cell population (right) in inguinal LN cells were analysed by flow cytometry using CD4- and B220-specific antibodies (WT, KO: n=8 each). *P<0.05 and **P<0.01. Student’s t-test. (d) The sera at day 42 after primary immunization were collected, and the IIC-specific IgG level was determined by ELISA (WT: n=10 and KO: n=9). ***P<0.001. Student’s t-test. (e) At 7 days after immunization, inguinal LN cells were cultured with IIC (0, 100, 200 μg ml−1). Then IIC-specific proliferative response was measured by [3H]TdR incorporation (WT, KO: n=8 each). Student’s t-test. (f) Incidence (left) and severity score (right) of CAIA under mild conditions (WT, KO: n=9 each). *P<0.05. χ2-test and Mann–Whitney U-test. All data were reproduced in another independent experiment with similar results. Average and s.e.m. are shown.
We also analysed susceptibility of C1qtnf6−/− mice to CAIA6, in which antibodies against IIC were directly injected to induce arthritis. We found that C1qtnf6−/− mice were much susceptible to CAIA than WT mice (Fig. 1f). Because CAIA is independent from antibody production, these results suggest that the effect of CTRP6 on the autoantibody-induced inflammation is important rather than on the production of antibodies.
C1qtnf6 Tg mice are refractory to the development of CIA
Next, we generated C1qtnf6 Tg mice carrying mouse C1qtnf6 under the control of the CAG promoter26 to examine the effects of excess CTRP6. Serum CTRP6 levels were ∼2.5 times higher in C1qtnf6 Tg mice than in non-Tg littermates (Supplementary Fig. 4a–d). Heterozygous C1qtnf6 Tg mice were born in the expected Mendelian ratio (WT:C1qtnf6 Tg=290 (53.5%):252 (46.5%), P=0.273), were fertile and showed no obvious abnormalities including the renal function (Supplementary Fig. 4e).
We examined the susceptibility of C1qtnf6 Tg mice to CIA, using a higher concentration of M. tuberculosis (2.5 mg ml−1) in CFA to induce severe arthritis. The arthritic severity score was significantly lower in C1qtnf6 Tg mice than in WT mice, although the incidence was similar (Fig. 2a). Histology of the joints of C1qtnf6 Tg mice exhibited milder pathological changes than that of WT mice (Fig. 2b,c). Serum IIC-specific IgG levels were also decreased in these C1qtnf6 Tg mice (Fig. 2d). Furthermore, C1qtnf6 Tg mice were refractory against the induction of CAIA compared with WT mice (Fig. 2e). These results suggest that high concentration of CTRP6 can suppress the development of arthritis.
(a) Incidence (upper) and severity score (lower) of CIA (WT: n=12, Tg: n=11). *P<0.05. χ2-test and Mann–Whitney U-test. (b,c) Histopathology (b) and histological score (c) of the ankle joints at day 42 after primary immunization (WT, Tg: n=9 each). Haematoxylin and eosin staining. Tibia, talus and navicular bone are represented as Tib, Tal and Nav, respectively. Bone destruction by pannus formation into navicular bone associated with the presence of osteoclasts and fibroblasts is seen in the WT joint. Scale bar, 300 μm. One of representative histologies is shown. **P<0.01. Student’s t-test. (d) IIC-specific IgG levels at day 42 after primary immunization (WT: n=12, Tg: n=11). *P<0.05. Student’s t-test. These results were reproduced in another independent experiment with similar results. (e) Incidence (upper) and severity score (lower) of CAIA under severe conditions. These data were combined from two independent experiments (WT, Tg: n=7 each). *P<0.05. χ2-test and Mann–Whitney U-test. Average and s.e.m. are shown.
CTRP6 specifically inhibits complement AP
Interestingly, we found that the concentrations of C3a and C5a in the plasma of IIC-immunized mice were increased in C1qtnf6−/− mice compared with WT mice (Fig. 3a). Furthermore, the deposition of C3b in the joints was increased in C1qtnf6−/− mice (Fig. 3b,c). In contrast, the deposition of C3b in the joints was lower in C1qtnf6 Tg mice than in WT mice (Fig. 3d,e). These results suggest that CTRP6 regulates complement activation.
(a) Plasma samples before (pre) and 7 days after (IIC) IIC immunization were collected, and C3a (upper) and C5a (lower) levels were measured by ELISA (WT, KO: n=8 each). *P<0.05. Student’s t-test. The data were reproduced in another independent experiment with similar results. (b,c) Cryostat sections of the WT and C1qtnf6−/− ankle joints at day 42 after primary immunization were stained with anti-C3b antibody and 4′,6-diamidino-2-phenylindole (DAPI) (nucleus). The photomicrographs were taken with fluorescence and differential interference contrast (DIC) optics. Scale bar, 20 μm. One of representative histologies is shown. The relative fluorescence intensity (RFI) of C3b (c) was determined by ImageJ (WT, KO: n=6 each). ***P<0.001. Student’s t-test. (d,e) Cryostat sections of WT and C1qtnf6 Tg ankle joints at day 42 after primary immunization were stained with anti-C3b antibody and DAPI (nucleus). The photomicrographs were taken with fluorescence and DIC optics. Scale bar, 50 μm. One of representative histologies is shown. The relative fluorescence intensity of C3b (e) was determined by ImageJ (WT, Tg: n=7 each). ***P<0.001. Student’s t-test. Average and s.e.m. are shown.
Then, we examined the effects of CTRP6 deficiency on three complement pathways in vitro. Plates coated with the ovalbumin (OVA)/anti-OVA immune complex, mannan and lipopolysaccharide (LPS) were used to activate the CP, LP and AP, respectively. Sera were diluted with GVB++ buffer containing Ca2+ for CP and LP assays, while sera for determination of AP activity were diluted with GVB/Mg2+EGTA to eliminate Ca2+, because the AP is Ca2+ independent. We found that AP activity was specifically enhanced in C1qtnf6−/− sera compared with WT sera (Fig. 4a). Furthermore, MAC formation under AP activation conditions was enhanced in C1qtnf6−/− mouse sera (Supplementary Fig. 5a). In contrast, C1qtnf6 Tg mouse serum specifically suppressed AP activation (Fig. 4b; Supplementary Fig. 5b). C3 and factor B levels were comparable in WT, C1qtnf6−/− and C1qtnf6 Tg mouse sera (Supplementary Fig. 5c). These results suggest that CTRP6 specifically suppresses AP activation.
(a–c) Complement activation was determined by C3b deposition. (a) The CP, LP and AP in 10% WT and C1qtnf6−/− mice serum were activated by the OVA/anti-OVA immune complex, mannans and LPS, respectively (WT, KO: n=8 each). *P<0.05, **P<0.01 and ***P<0.001. Student’s t-test. (b) Complement activation in 10% WT and C1qtnf6 Tg mouse serum (WT, Tg: n=8 each). (c) The AP in 10% C1qtnf6−/− serum was activated by LPS in the presence of rhCTRP6 (n=8). (d) AP C3 convertase activity. RhCTRP6 was incubated with a mixture of human C3, factor B and factor D. The reaction mixtures were subjected to SDS–PAGE and were visualized by CBB staining (upper). AP C3 convertase activity (lower) was calculated by the intensity of the Bb protein band. Images have been cropped for presentation. Full-size images are presented in Supplementary Fig. 5g. (e) After incubation of human C3(H2O) with human factor B and rhCTRP6, C3 was immunoprecipitated with anti-C3 antibody, and immunoprecipitates were subjected to SDS–PAGE. After staining with CBB (upper), the factor B band intensity (lower) was quantified. Images have been cropped for presentation. Full-size images are presented in Supplementary Fig. 5h. (f) RhCTRP6 (+) or dilution buffer (−) was added to C1qtnf6−/− serum, and rhCTRP6-associated proteins were immunoprecipitated with anti-CTRP6 antibody. Immunoprecipitates were subjected to SDS–PAGE, and C3 and CTRP6 were detected with anti-C3 and anti-CTRP6 antibody, respectively. Images have been cropped for presentation. Full-size images are presented in Supplementary Fig. 5i,j. (g) C3–CTRP6 interaction was detected by surface plasmon resonance analysis. Human C3 (0, 15.625, 31.25, 62.5, 250 and 500 nM) was flowed over a rhCTRP6-immobilized sensor chip. The response is expressed in resonance response units (RUs). All data were reproduced in another independent experiment. Average and s.e.m. are shown.
Then, we directly analysed the effect of CTRP6 on AP activation in vitro. Because C1q domain of human and mouse CTRP6 are highly homologous and CTRP6 mainly forms oligomers in the serum (Supplementary Fig. 5d), we analysed the effect of monomeric exogenous recombinant human CTRP6 (rhCTRP6) on the complement activity of mouse sera. C1qtnf6−/− sera were used to avoid possible influence of endogenous CTRP6. Results showed that rhCTRP6 specifically inhibited AP activation in a dose-dependent manner (Fig. 4c; Supplementary Fig. 5f).
On the basis of these findings, we investigated in further detail the effects of CTRP6 on AP activation. Upon activation of the AP, spontaneously generated C3(H2O) forms a complex with factor B, followed by cleavage of factor B by factor D to generate C3(H2O)Bb, a C3 convertase, and Ba. C3 is composed of C3α and C3β; C3 is cleaved to C3a and C3b (consisting of C3α′ and C3β) by C3 convertase (Supplementary Fig. 6)27. After incubation of human C3, factor B and factor D in the presence of various concentrations of rhCTRP6 in vitro, the reaction mixture was subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and visualized by Coomassie brilliant blue (CBB) staining (Fig. 4d; Supplementary Fig. 5g). The result showed that rhCTRP6 inhibited proteolytic activation of factor B in a dose-dependent manner, indicating that CTRP6 suppressed C3 convertase formation. Next, we examined the effect of CTRP6 on the formation of the C3(H2O)–factor B complex, the first step of C3 tick-over. After incubating a mixture of human C3(H2O), human factor B and rhCTRP6, C3-containing protein complexes were immunoprecipitated using anti-C3 antibodies. Co-immunoprecipitation of factor B decreased as a function of rhCTRP6 dose (Fig. 4e; Supplementary Fig. 5h), suggesting that CTRP6 competitively inhibits factor B binding to C3(H2O). Furthermore, exogenously added rhCTRP6 co-precipitated with C3 from C1qtnf6−/− serum (Fig. 4f; Supplementary Fig. 5i,j). Direct binding of rhCTRP6 to human C3(H2O), but not to C3b, was shown by surface plasmon resonance (SPR) analysis (KD=1.1 × 10−6) (Fig. 4g; Supplementary Fig. 5k). Furthermore, we examined the association between CTRP6 and factor H, because factor H also binds C3(H2O)28 and adiponectin, one of the CTRP family associated with factor H to modulate the AP activation29. However, CTRP6 did not bind factor H nor influence its activity (Supplementary Fig. 5l,m). These results demonstrated that CTRP6 regulates AP activation by binding to C3(H2O).
C1qtnf6 deficiency enhances the Arthus reaction and EAE
The Arthus reaction is an immune complex-mediated type-III hypersensitivity that depends on complement activation24. To confirm that CTRP6 is a complement inhibitor, we examined the sensitivity of C1qtnf6−/− mice to an IgG-mediated reverse passive Arthus (RPA) reaction. We found that vascular permeability was significantly enhanced in C1qtnf6−/− mice compared with WT mice (Supplementary Fig. 7a). These results support the notion that CTRP6 is a regulator of the complement system.
MS is an autoimmune disease of the central nervous system. Studies of EAE, an experimental model of MS, have suggested that the AP, but not the CP or LP, is important for the pathogenesis30,31. Another recent study has also suggested the involvement of complement activation in MS pathogenesis8. Therefore, we investigated whether C1qtnf6 deficiency affected the development of EAE. The severity score was much higher in C1qtnf6−/− mice than in WT mice, although the incidence of disease was similar between the two strains (Supplementary Fig. 7b). These results provide further support for the notion that CTRP6 is a novel regulator of the complement system.
CTRP6 is effective for the treatment of CIA
Next, we examined whether CTRP6 can treat established arthritis. We immunized DBA/1J mice with IIC/CFA (M. tuberculosis 0.5 mg ml−1), followed by a booster injection with IIC/CFA on day 21. Starting on day 28, we performed daily injections of rhCTRP6 into the articular cavity of the knee joints of affected mice (rhCTRP6 into left leg; PBS or rhCTRP9 into right as a control), and evaluated the swelling of the ankle joints. As shown in Fig. 5a and Supplementary Fig. 8, ankle joint swelling was promptly ameliorated by the injection of rhCTRP6 to the knee joint, whereas the ankle joints of the PBS- or rhCTRP9-injected side still remained swollen. In the rhCTRP6-treated joints of the WT mice, messenger RNA (mRNA) expression levels of Il1b, Tnfa and F4/80 were decreased, whereas Il10 mRNA level did not change (Fig. 5b). Histology of joints revealed that PBS-injected joints were destroyed, but rhCTRP6-injected joints were normal (Fig. 5c). C3b deposition was decreased in the rhCTRP6-injected joints relative to the PBS-injected joints (Fig. 5d). Next, we injected rhCTRP6 to arthritic C3−/− mice to investigate whether CTRP6 can treat arthritis in a complement-independent fashion. In contrast, rhCTRP6 failed to treat arthritis in C3−/− mice (Fig. 5e), suggesting that C3 is involved in the manifestation of CTRP6 action. Messenger RNA expression levels of Il1b, Tnfa and F4/80 were not changed in these rhCTRP6-treated C3−/− mice. However, Il10 mRNA levels were increased (Fig. 5f). These results suggest that CTRP6 is effective for the treatment of autoimmune arthritis.
(a,b) RhCTRP6 or PBS as a control was daily injected into the articular cavity of the left (rhCTRP6) or right (rhCTRP9) knee joint of CIA-induced DBA/1J mice from day 28 (n=11). (a) Incidence (left) and severity score (right) of CIA. *P<0.05. χ2-test and Mann–Whitney U-test. (b) Messenger RNA expression in the joints was measured by semi-quantitative PCR analysis. *P<0.05, **P<0.01, and ***P<0.001. Student’s t-test. (c) Histology (left) and histological score (right) of the ankle joints at day 42. Haematoxylin and eosin. Tibia, talus and navicular are represented as Tib, Tal and Nav, respectively. Scale bar, 300 μm. One of representative histologies is shown (n=6). **P<0.01. Student’s t-test. (d) Cryostat sections of the joints were stained with anti-C3b antibody, isotype IgG and 4′,6-diamidino-2-phenylindole (nucleus) (left). One of representative histologies is shown. The photomicrographs were taken with fluorescence and differential interference contrast (DIC) optics. The relative fluorescence intensity of C3b (right) was determined by ImageJ (n=4). Scale bar, 50 μm. ***P<0.001. Student’s t-test. (e,f) RhCTRP6 or rhCTRP9 as a control was daily injected into the articular cavity of the left (rhCTRP6) or right (rhCTRP9) knee joint of CIA-induced C3−/− mice from day 21 (n=10). (e) Incidence (left) and severity score (right) of CIA in C3−/− mice. χ2-test and Mann–Whitney U-test. (f) Messenger RNA expression in the joints of C3−/− mice after treatment with rhCTRP6 are shown relative to those of control-treated mice were measured by semi-quantitative PCR analysis. *P<0.05. Student’s t-test. Average and s.e.m. are shown.
CTRP6 is expressed in synovial cells of RA patients
Finally, we investigated CTRP6 expression in RA patients. We found that CTRP6 concentration was increased in sera of RA patients compared with that of healthy controls (Fig. 6a). CTRP6 was detected in synovial lining cells of RA patients (Fig. 6b). Double-immunostaining using RA synovial tissues showed that CTRP6 expression was localized to Hsp47+ synovial cells32 (Fig. 6c–f; Supplementary Fig. 9). Furthermore, we found that C1qtnf6 expression in primary fibroblast-like synoviocytes was enhanced by IL-1α stimulation (Fig. 6g). These results indicate that CTRP6 expression is elevated in synovial cells in RA patients.
(a) The CTRP6 levels in the serum of healthy controls (HC) and RA patients (RA) were measured by ELISA (HC: n=22, RA n=30). *P<0.05. Student’s t-test. (b) Synovium from a RA patient was stained with anti-CTRP6 antibody or isotype IgG using diaminobenzidine tetrahydrochloride staining. Scale bar, 50 μm. (c–f) Cryostat sections of synovia from RA patients were stained with anti-CTRP6 antibody and 4′,6-diamidino-2-phenylindole (nucleus), and either anti-CD3 antibody (c), or anti-CD20 antibody (d), or anti-CD68 antibody (e), or anti-Hsp47 antibody (f). Scale bar, 20 μm. (g) Expression of C1qtnf6 in primary fibroblast-like synoviocytes stimulated with IL-1α (n=3) is shown. *P<0.05. Student’s t-test. Average and s.e.m. are shown.
Discussion
Because C3a and C5a have chemotactic, anaphylatoxic and IgG production-promoting activity, and the MAC has strong cytolytic activity, complement activity must be strictly controlled under physiological conditions. When this control fails, excess or inappropriate activation of a complement pathway can cause multiple human diseases1,8,33. Thus, complement regulatory factors are important for the homeostasis of the complement system. In this report, we have shown that CTRP6 is a novel endogenous regulator of the AP and the AP was excessively activated in C1qtnf6−/− mice. CTRP6 efficiently suppresses the AP ‘tick-over’ by inhibiting factor B binding to C3(H2O) in a competitive manner. It is known that AP activation is also regulated by factor I and factor H; both regulate the ‘AP amplification loop’ (Supplementary Fig. 1), and factor H also blocks the tick-over reaction. Factor I, in cooperation with factor H, cleaves C3b to generate inactive C3b (iC3b). Factor H also accelerates decay of C3 convertase (C3bBb) by dissociating Bb34. Factor H-deficient (Cfh−/−) mice spontaneously develop membrane-proliferative glomerulonephritis due to C3 dysregulation, but factor I-deficient (Cfi−/−) mice do not develop glomerulonephritis, in spite of C3 dysregulation9,10. C1-inhibitor-deficient (C1inh−/−) mice show increased vascular permeability35. Furthermore, deficient mice of the complement receptor 1-related gene/protein-y (Crry) gene, which regulates CP and AP activation, are 100% embryonic lethal due to spontaneous complement activation36.
It is reported that factor H also interacts with C3(H2O) via the residues Glu-744 and Glu-747 of C3 (ref. 37). However, factor B–C3(H2O) interaction, which is inhibited by CTRP6, occurs at different residues from the factor-H-binding site38. Furthermore, CTRP6 did not bind factor H nor influence its activity (Supplementary Fig. 5l,m). Thus, the inhibitory mechanism of CTRP6 is different from that of factor H. Although the binding affinity between C3(H2O) and CTRP6 was relatively low (KD=1.1 × 10−6), the real binding affinity may be greater than this because CTRP6 forms oligomers in the serum (Supplementary Fig. 5d). Because the binding of factor B and C3(H2O) initiates AP activation, the regulation of this step should be critically important for the AP activation. Actually, we found that the frequency of homozygotes from the mating between C1qtnf6 F1 heterozygotes on the (129P2/Ola × C57BL/6) background was less than that expected by the Mendelian ratio, partially resembling Crry−/− mice36. This observation suggests a beneficial role of CTRP6 in the development of fetus, consistent with the high levels of expression in the placenta. Furthermore, aged C1qtnf6−/− mice produced significantly higher levels of autoantibodies, probably reflecting the increase of C3a and C5a, which can promote IgG production1, although we did not observe any renal failure caused by immune complexes. Thus, CTRP6 plays important roles in the regulation of the complement system under the physiological conditions. Although adiponectin, another CTRP family member, also suppresses the activation of the AP, this occurs through the activation of factor H29, a different mechanism from CTRP6.
AP activation is implicated in the development of RA and other inflammatory diseases5,6,7,8,39. IgGs in immune complexes with agalactosylated complex type N-glycans, which are increased in sera of RA patients40 as well as in RA models41, can activate the AP42. In this report, we showed that the development of CIA, in which complement activation plays a deleterious role39, was greatly exacerbated in C1qtnf6−/− mice, and ameliorated in C1qtnf6 Tg mice. These findings suggest that CTRP6 is involved in the regulation of arthritis development by suppressing the AP. Although we observed changes of anti-IIC levels in C1qtnf6−/− mice and C1qtnf6 Tg mice upon induction of CIA, these are secondary effects of AP activation/suppression and are not responsible for their severity score changes, because the severity scores in CAIA model, in which antibody production is not necessary, also changed in C1qtnf6−/− mice and in C1qtnf6 Tg mice.
The involvement of the AP in the development of RA was also suggested in other RA models. The development of arthritis in K/BxN serum-transferred mice, a model in which autoantibodies against glucose-6-phosphate isomerase play an important role, is suppressed by the deficiency of factor B, C3, C5 or C5a, but not by C1q or MBP-A, suggesting the involvement of the AP in the pathogenesis5. The development of arthritis in SKG mice, which carry a mutation in the T-cell receptor component ZAP70, is suppressed by the deficiency of C5aR7. In that report, the authors showed that C5a signalling induces the development of Th17 cells through induction of cytokines such as IL-6, IL-1β, IL-23 and granulocyte–macrophage colony-stimulating factor in macrophages7. Because IL-17 can enhance antibody production43, the promotion of Th17 cell differentiation also explains why much higher levels of anti-IIC IgG were produced in C1qtnf6−/− mice than WT mice upon immunization with IIC. Furthermore, it was recently reported that absence of C3a and C5a signalling can promote Treg cell differentiation by inducing TGF-β from T cells44, implying that excess AP activation may suppress the development of Treg cells. Therefore, excess activation of the complement system not only directly induces inflammation by recruiting macrophages and neutrophils but also induces autoimmunity and inflammation by enhancing Th17 cell differentiation, suppressing Treg cell differentiation and enhancing antibody production. Thus, CTRP6 may be an important regulator of inflammation by preventing excess complement activation under pathological conditions.
We have shown that CTRP6 injection is effective for the treatment of CIA. When rhCTRP6 was injected into the articular cavity of the left knee, clear suppressive effect on the ankle swelling of the same leg was observed, indicating that knee-joint-injected rhCTRP6 diffuses into the ipsilateral ankle joint. We used a contralateral leg as the control, which was injected with PBS or CTRP9 to the knee joint. Although the effect of rhCTRP6 to the contralateral leg was only marginal, the severity score of the control ankle joints could be lower than those of rhCTRP6-untreated mice due to the possible contribution of systemic effects. C3−/− mice were refractory against the CTRP6 treatment, indicating that the inhibition of the AP is responsible for the therapeutic effect. We observed that rhCTRP6 enhanced IL-10 production from WT and C1qtnf6−/− bone marrow (BM)-derived macrophages (Supplementary Fig. 10), consistent with a report that shows IL-10 induction by CTRP6 from Raw264.7 macrophages45. Importantly, Il10 mRNA expression was still elevated in the CTRP6-treated joints of C3−/− mice (Fig. 5e). These observations suggest that the therapeutic effect of CTRP6 on CIA mainly depends on its complement inhibitory activity, but not on the IL-10-inducing activity. In support for this notion, it was reported that the disease activity of RA and circulating levels of IL-10 are not correlated46.
CTRP family including adiponectin is known as an adipokine. The CTRP family regulates systemic energy homeostasis via adipoR1, adipoR2 and unknown receptors47. And we have already reported that CTRP3 is important for the development of autoimmune disease, but is not a complement regulator19,21. Thus, it remains a possibility that CTRP6 has complement-independent functions.
Suppression of the complement system is an attractive strategy for the treatment of inflammatory diseases involving excess complement activation. Indeed, an antibody against C5a (eculizumab) has been successfully used for the treatment of paroxysmal nocturnal hemoglobinuria48. The involvement of the complement system is also suggested in other diseases. We have shown that EAE, in which the involvement of the AP has been implicated8, is significantly enhanced in C1qtnf6−/− mice. Furthermore, C1QTNF6 locus is a susceptibility locus associated with autoimmune diseases including RA and type-1 diabetes49,50,51. Together, these observations suggest that CTRP6 may play important roles not only in RA but also in other inflammatory diseases that involve AP activation, such as MS, type-1 diabetes52, age-related macular degeneration, systemic lupus erythematosus and glomerulonephritis1,8,53. Thus, CTRP6 is a promising candidate of the medicine for the treatment of RA and other inflammatory diseases in humans.
Methods
Reverse transcription and real-time PCR
Total RNA was extracted using Sepasol-RNA I Super (Nacalai Tesque, Japan). RNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). Reverse transcription (RT)-PCR using Taq (TaKaRa, Japan) and iCycler System (Bio-Rad, USA) was performed with the sets of primers described in Supplementary Table 1. Quantitative real-time RT-PCRs were performed using SYBR Premix Ex Taq (TaKaRa) and an iCycler System (Bio-Rad) with the sets of primers described in Supplementary Table 1.
Mice
C1qtnf6−/− mice were generated using E14.1 embryonic stem (ES) cells, and C1qtnf6 Tg mice were generated by pronuclear microinjection into C57BL/6J mouse embryos and were used as heterozygotes as described in the following sections. C3−/− mice (C57BL6/J background) were obtained from Jackson laboratories. Mice were kept under specific pathogen-free conditions in the clean rooms at the Center for Experimental Medicine and Systems Biology, Institute of Medical Science, University of Tokyo, and the Research Institute for Biomedical Sciences, Tokyo University of Science. All experiments were approved by the committee of Life Science Research Ethics and Safety of the University of Tokyo and the Animal Care and Use Committee of Tokyo University of Science, and were conducted according to the institutional ethical guidelines for animal experiments and safety guidelines for gene manipulation experiments.
Generation of C1qtnf6−/− mice
The C1qtnf6−/− allele was created by homologous recombination in E14.1 ES cells. Homology arms of the C1qtnf6 gene-targeting vector were amplified from genomic DNA of E14.1 ES cell by PCR methods using the primer set 1 and 2 for the 5′ arm and 3′ arm, respectively (Supplementary Table 2). The lengths of the 5′ and 3′ arms are 3.5 and 6.9 kb, respectively. The targeting vector was constructed by replacing the genomic locus containing the third exon of the C1qtnf6 gene, which encodes the C1q domain, with a neomycin-resistance gene (Neor) under the control of a PGK1 promoter for positive selection. A diphtheria toxin A gene under the MC1 promoter was ligated to the 3′ end of the targeting vector for negative selection. In addition, the stop codon and HindIII sequence for screening were introduced into exon 2 in the 5′ homology arm of the targeting vector. The targeting vector was electoroporated into ES cells and selected in G418 (Nacalai Tesque). We screened 223 neomycin-resistant ES clones by PCR and Southern blotting. The first screening was performed by PCR using the primer set 3 (Supplementary Table 2). For positive clones from the PCR screening, we confirmed correct homologous recombination by Southern blotting using 5′ and 3′ flanking probes. Genomic DNA of the targeting clones was digested using HindIII and EcoRV, respectively, for detection by the 5′ and 3′ probe. The DNA fragment for the 5′ probe was amplified using the primer set 4 and 5 for 5′ and 3′ probe, respectively (Supplementary Table 2). As a result of this screening, we obtained one targeted clone (clone ID: 3C2) (targeting efficiency: 0.5%). Using this clone, chimeric mice were generated by an aggregation method modified from previous reports54,55. The contribution of ES cells to chimeric mice was checked by coat colour. To generate heterozygous offspring, male chimeric mice were mated with C57BL/6J female mice, and (129P2/Ola × C57BL/6) F1 progeny were obtained. In this report, we used mice of the C57BL/6 background. Mice were backcrossed with C57BL/6J mice for five generations using the high-speed congenic strategy25. The proportion of C57BL/6J homozygous loci in male mice of the N5 generation was 100% (84 markers). The genotyping of C1qtnf6−/− mice were carried out using the following primers: WT sense primer 1, mutant sense primer 1 and common antisense primer 1 (Supplementary Table 3). The common primer and the WT primer were used to detect the WT allele (686 bp), and the common primer and mutant primer were used to detect the mutant allele (454 bp).
Generation of C1qtnf6 Tg mice
An EcoRI-flanked full-length mouse C1qtnf6 complemntary DNA fragment was obtained by RT-PCR using the primer set 6 (Supplementary Table 2). This fragment was introduced into the EcoRI site of the pCXN2 plasmid26. The transgene was designed for ectopic expression of C1qtnf6 under control of the CAG promoter. We confirmed by direct sequencing that the fusion gene contained no errors. CAG-C1qtnf6-polyA fragments were digested by BamHI and PvuI to eliminate the plasmid backbone sequence. A purified CAG-C1qtnf6-polyA fragment was microinjected into the pronuclei of fertilized eggs of C57BL/6J mice (CLEA Japan, Japan). Viable embryos were transferred into the oviducts of pseudopregnant female ICR mice (Japan SLC, Japan), and pups were obtained. Mice carrying the CAG-C1qtnf6 transgene were identified by PCR screening using the primer set 7 (Supplementary Table 2). Then, we confirmed Tg founder mice by Southern blot analysis of tail DNA, using a probe for the HindIII/EcoRI site in the C1qtnf6 sequence. The Tg probe was amplified by PCR using the primer set 8 (Supplementary Table 2). The genotyping of C1qtnf6 Tg mice was carried out using the following primers: WT sense primer 2, mutant sense primer 2 and common antisense primer 2 (Supplementary Table 3). The Tg mice were used as heterozygotes.
Titration of CTRP6
To measure CTRP6 levels in serum, we collected mouse sera and diluted 10 times with PBS (pH 7.4). Plates (Nunc, Denmark) were coated with polyclonal rabbit antibodies against human and mouse CTRP6 (AnaSpec, USA, 54561, 1:1,000 dilution). After incubation with diluted serum (50 μl) for 60 min, biotin-labelled polyclonal rabbit antibodies against human and mouse CTRP6 (AnaSpec, 54562, 1:1,000 dilution) were added, and the absorbance at 450 nm was inspected after incubation with avidin-conjugated horseradish peroxidase (HRP). RhCTRP6 (BioVendor, Czech Republic) was used as the standard.
Titration of autoantibodies
We collected serum from aged mice (male 129P2_Ola × C57BL/6J F1 background at 1 year of age) and titrated autoantibodies, such as ANA and RF. To measure ANA, we performed enzyme-linked immunosorbent assay (ELISA) with plates coated with nuclear antigens and HRP-conjugated polyclonal goat antibodies to mouse IgG using the Autoimmune ELISA kit (Alpha Diagnostic, USA, 5210). To measure RF, we performed ELISA with plates coated with heat-denatured rabbit IgG (Santa Cruz, USA, sc-2027, 1:8 dilution) and alkaline phosphatase-conjugated polyclonal rabbit antibodies to mouse IgG (Zymed, USA, 62–6622, 1:1,000 dilution) and IgM (Santa Cruz, sc-2070, 1:1,000 dilution).
Flow cytometric analysis
For flow cytometry, we harvested thymus, spleen and LN from mice (male 129P2_Ola × C57BL/6J F1 background at 8 weeks of age) and stained cells with a 100-fold dilution of phycoerythrin-Cy7-, fluorescein isothiocyanate (FITC)-, APC-Cy7-, Pacific-Blue-, APC- and phycoerythrin-conjugated monoclonal antibodies (mAbs). We purchased rat or hamster monoclonal antibodies to mouse CD4 (GK1.5), CD3 (145-2C11), B220 (RA3–6B2) and CD11b (M1/70) from Biolegend (USA) and CD8 (53-6.7) from eBioscience (USA) and CD11c (HL3) from BD Pharmingen (USA). We performed cell-surface staining according to standard techniques, and we analysed stained cells using a FACS Canto II cytometer and either CellQuest (BectonDickinson, USA) or FlowJo software (Tree Star, USA).
Thymus-dependent and -independent antibody production
We used 2,4,6-trinitrophenyl (TNP)-keyhole limpet haemocyanin (KLH) (Biosearch Technologies, USA) and TNP-AminoEthylCarboxyMethyl-FICOLL (Biosearch Technologies) as a thymus-dependent antigen and thymus-independent antigen, respectively. To examine antibody production against thymus-dependent antigens, mice (female C57BL/6J background at 8 weeks of age) were immunized intraperitoneally with 100 μl of 100 μg TNP(29)-KLH (Biosearch Technologies) emulsified with Imject Alum (Thermo Scientific, USA). On day 21, mice were given an intraperitoneal booster injection with the same amount of TNP-KLH/Imject Alum. On days 0, 14 and 30, the serum was collected and TNP-specific IgG1 and IgM were determined by ELISA using 20 μg ml−1 TNP(30)-bovine serum albumin (BSA)-coated plates and alkaline phosphatase-conjugated polyclonal rabbit antibodies to mouse IgG1 and IgM (Zymed, 61–0122, 1:1,000 dilution and 62–6822, 1:1,000 dilution).
To elucidate the antibody production against thymus-independent antigens, mice (female C57BL/6J background at 8 weeks of age) were immunized intraperitoneally with 100 μl of 100 μg TNP(65)-AminoEthylCarboxyMethyl-FICOLL (Biosearch Technologies). On days 0 and 7, the serum was collected and TNP-specific IgG3 and IgM were determined by ELISA using 20 μg ml−1 TNP(30)-BSA-coated plates and alkaline phosphatase-conjugated polyclonal rabbit antibodies to mouse IgG3 and IgM (Zymed, 1100-04, 1:1,000 dilution and 62–6822, 1:1,000 dilution).
Titration of protein concentration in urine
Urine was collected from mice (male C57BL/6J background at 1 year of age) and total protein levels were measured using a bicinchoninic acid assay kit (Thermo Scientific).
Collagen-induced arthritis
On day 0, mice (female C57BL/6J background at 6–8 weeks of age) were immunized intradermally near the base of the tail with 100 μl of 2 mg ml−1 chicken IIC (Sigma, USA) emulsified with CFA composed of IFA (Thermo Scientific) plus M. tuberculosis H37Ra (Difco, USA; 1.65 or 2.5 mg ml−1)22. On day 21, mice were given an intradermal booster injection near the previous injection sites with the same amount of IIC/CFA. We judged the development of arthritis in C57BL/6J mice by macroscopic evaluation. Arthritis development in each paw was graded as follows: 0, no change; 1, mild swelling; 2, obvious joint swelling; 3, severe joint swelling and ankylotic changes (maximum of 12 points for individual mice)56.
To examine the therapeutic effect of CTRP6 for autoimmune arthritis, we injected rhCTRP6 to arthritic mice. DBA/1J mice (female, 6–8 weeks of age) were immunized with 100 μl of 2 mg ml−1 IIC emulsified with CFA (Difco: 0.5 mg ml−1 M. tuberculosis H37Ra) on day 0 and on day 21 as described above. Then, 300 ng/30 μl rhCTRP6 (Aviscera Bioscience, USA) or control (30 μl PBS or 300 ng/30 μl rhCTRP9: Aviscera Bioscience) was injected every day into the articular cavity of the left (CTRP6) or right (control) knee joint from day 28. We judged the development of arthritis in DBA/1J mice by macroscopic evaluation. Arthritis development in each paw was graded as follows: 0, no change; 1, erythema and mild swelling confined to the tarsal joints; 2, erythema and mild swelling extending from the tarsal joint to digit; 3, erythema and moderate swelling extending from metatarsal joints; 4, erythema and severe swelling encompassing the ankle, foot and digits, or ankylosis of the limb (maximum four points for one leg of individual mice)57. WT and C3−/− mice (C57BL/6 background, female, 6–8 weeks of age) were immunized with 200 μl of 2 mg ml−1 IIC emulsified with CFA composed of IFA (Thermo Scientific) plus M. tuberculosis H37Ra (Difco; 0.5 mg ml−1) on day 0 and on day 14 as described above. RhCTRP6 (Aviscera Bioscience) or rhCTRP9 (Aviscera Bioscience) (300 ng/30 μl for each) as a control was injected every day into the articular cavity of the left (CTRP6) or right (control) knee joint from day 21. We checked possible aggregation of rhCTRP6 protein by gel filtration chromatography and used the monomer form rhCTRP6, which is free from any aggregation. We judged the development of arthritis by macroscopic evaluation as described above56.
Histopathology
Mice were killed under ether anaesthesia, and their hindlimbs were processed for histopathology. Whole-ankle joints were fixed in 10% formalin in 1 mM phosphate buffer, pH 7.2, decalcified in 10% formic acid and embedded in paraffin. Serial sections of 2–3 μm thickness were taken sagittally through the talus and stained with haematoxylin and eosin for examination by light microscopy. The lesions, including the calcaneus bone and anterior and posterior synovial tissues at the ankle joints, were evaluated histopathologically. Histological severity of each joint was graded as follows: 0, normal; 1, thickening and proliferation of the synovial lining, with slight inflammatory cell infiltration; 2, grade 1 changes plus granulomatous lesions in the synovial sublining tissue; 3, grade 2 changes plus pannus formation and bone destruction. Arthritis index of the ankle joint was estimated from the average grade of talus and around bones including tibia and calcaneum of each mouse.
For immunostaining, limbs were frozen and embedded in special cryomedium SCEM (Leica Microsystems, Japan) and cryosectioned (5 μm thickness) onto a cryofilm (Leica Microsystems). The sections were fixed in cold acetone for 5 min and blocked with 20% goat serum (Wako, Japan)/1% BSA (Sigma) in PBS at room temperature for 1 h. Rat anti-C3 antibody (Abcam, UK, ab11862, 1:1,000 dilution), rat anti-IgG2a (R&D Systems, USA, 344701, 1:1,000 dilution) and Alexa Fluor 488 anti-rat IgG (Invitrogen, USA, A-11006, 1:1,000 dilution) were used for immunostaining. Nuclei were stained with 4′,6-diamidino-2-phenylindole. Relative fluorescence intensity of C3b was determined by ImageJ. The slides were visualized on a Nikon A1Rsi confocal microscope (Nikon, Japan) operated by NIS-Elements software (Nikon).
IIC-specific antibody titration
For titration of IIC-specific antibodies in CIA, we collected serum samples from mice before primary immunization (pre) and on days 14, 28 and 42 after immunization and diluted them 1:100. We performed ELISA using 20 μg ml−1 IIC-coated plates and alkaline phosphatase-conjugated polyclonal rabbit antibodies to mouse IgM and IgG (Zymed, 62–6822, 1:1,000 dilution and 62–6522, 1:1,000 dilution).
IIC-specific LN cell response
LN cells were harvested from mice at day 7 after IIC immunization. LN cells were cultured in the absence or presence of 100 or 200 μg ml−1 denatured IIC for 72 h, followed by incorporation of [3H] thymidine (0.25 μCi ml−1) (Amersham, UK) for 6 h. Then cells were harvested with the Micro 96 cell harvester (Skatron, Norway), and radioactivity was measured with Micro Beta (Pharmacia Biotech, USA).
Collagen antibody-induced arthritis (CAIA)
CAIA was induced by injecting a mixture of five mouse monoclonal anti-IIC antibodies together with LPS (Chondrex, USA). Mice (female C57BL/6J background at 8 weeks of age) received an intravenous injection of 5 mg anti-IIC mAb on day 0, and i.p. injection of 50 μg (mild) or 100 μg (severe) LPS on day 3. We judged the development of arthritis by macroscopic evaluation. Arthritis score was determined as described in CIA58.
Titration of C3a and C5a
To measure C3a and C5a levels in plasma, we collected plasma from mice. We performed sandwich ELISA using the capture antibody-coated plates and detection antibodies against C3a and C5a (BD Pharmingen, C3a: BD558250, 1:1,000 dilution and BD558251, 1:1,000 dilution, C5a: BD558027, 1:1,000 dilution and BD558028, 1:1,000 dilution).
In vitro complement activation assay
Plates (Nunc) were coated with the OVA/anti-OVA immune complex (OVA: Sigma, 5 μg ml−1 and anti-OVA Ab: Millipore, Germany, AB1225, 1:500,000 dilution), 50 μg ml−1 mannan (Sigma) and 200 μg ml−1 LPS (Sigma) for assay of complement activation of CP, LP and AP, respectively59,60,61. Diluted mouse serum was incubated on plates at 37 °C for 1 h. We detected the deposition of C3b by rat mAb against mouse C3 (Abcam, ab11862, 1:1,000 dilution). The CP and LP activity was assayed in GVB++ buffer, and AP activity was assayed in GVB/Mg2+EGTA buffer. For reconstitution experiments, we assayed AP activity using C1qtnf6−/− serum in GVB/Mg2+EGTA buffer premixed with various concentrations of rhCTRP6 (BioVendor). In addition, we detected the formation of MAC by rabbit polyclonal antibody to mouse MAC (Abcam, ab55811, 1:1,000 dilution). Plates were coated with 50 μg ml−1 LPS, and we assayed the AP activity using C1qtnf6−/− mice serum in GVB/Mg2+EGTA buffer premixed with various concentrations of rhCTRP6.
C3 convertase activity assay
To elucidate the effect of CTRP6 on AP C3 convertase activity, 1 μg human C3 (Sigma), 1 μg human factor B (CompTech, USA), 100 ng human factor D (CompTech) and 0–100 ng rhCTRP6 (Aviscera Bioscience) in 15 μl of reaction buffer (2 mM CaCl2, 0.5 mM MgCl2, 20 mM Tris-HCl (pH 7.6)) were incubated at 37 °C for 1 h. The samples were subjected to SDS–PAGE and visualized by CBB staining. The inhibitory effect of CTRP6 on AP C3 convertase activity was calculated from the intensity of factor B protein band, measured using ImageJ. To investigate the association between CTRP6 and factor H, 1 μg C3, 1 μg factor B and 100 ng factor D with/without 100 ng rhCTRP6 and/or 1 μg factor H (CompTech) in 15 μl of reaction buffer were incubated at 37 °C for 1 h, and the reaction mixtures were subjected to SDS–PAGE and visualized by CBB staining.
Titration of C3 and factor B
C3 and factor B levels in sera were measured using an ELISA kit (C3: Abcam, ab157711 and factor B: USCN Life Science, USA, sE92011Mu).
Competitive binding assay
For measurement of competition between human CTRP6 and human factor B for binding to C3(H2O), a mixture of 20 μg C3(H2O); 20 μg factor B (provided by T. Fujita); 0, 20 or 100 μg rhCTRP6 (Aviscera Bioscience); 1.5 μg anti-C3 antibody (Abcam, ab11871); and 20 μl Protein A/G PLUS-Agarose (Santa Cruz) in 0.5 ml of a reaction buffer (2 mM CaCl2, 0.5 mM MgCl2, 20 mM Tris-HCl (pH 7.6)) was incubated at 37 °C for 2 h. After washing with PBS/0.05% Tween-20, samples were subjected to SDS–PAGE and visualized by CBB staining. Immunoprecipitated factor B was semi-quantified by the band intensity of factor B, which is normalized to the band intensity of C3β using ImageJ.
Immunoprecipitation and western blotting
We collected C1qtnf6−/− mouse serum and pre-cleared with Protein A/G Plus-Agarose (Santa Cruz). To immunoprecipitate CTRP6-associated proteins, 200 ng CTRP6 (BioVendor) or 10 mM acetic acid (control) was added to 1 ml pre-cleared C1qtnf6−/− mouse serum, and immunoprecipitated with 2 μg rabbit polyclonal antibody (Anaspec, 54561) to CTRP6. Immunoprecipitates were washed five times with PBS-1% Tween-20 and dissolved in SDS–PAGE running buffer (0.04 M Tris-HCl; 0.022 M glycine; 1% (w/v) SDS; pH 8.3). Aliquots of immunoprecipitate were separated by SDS–PAGE on 12.5% gels. Resolved proteins were transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 5% skim milk/Tris-buffered saline containing 0.1% Tween-20 (TBST) at room temperature for 1 h, followed by incubation overnight at 4 °C with rat mAb against C3 (Abcam, ab11862, 1:1,000 dilution) or rabbit polyclonal antibody against CTRP6 (Anaspec, 54562, 1:1,000 dilution). The membranes were then washed three times with TBST, followed by 1 h incubation at room temperature with HRP-conjugated goat polyclonal antibody against rat IgG (Zymed, 81–9520, 1:1,000 dilution). The membranes were washed six times with TBST and visualized using the ECL Prime Western Blotting Detection System (GE Healthcare, Japan).
SPR-based interaction assay
Each human recombinant protein (CTRP6, Aviscera Bioscience; C3b, Alpha Diagnostic) were linked to CM5 chips via amino coupling. The coupling was performed according to the standard N-ethyl-N′-(3-dimethyl aminopropyl)-carbodiimide hydrochloride/N-hydroxysuccinimide procedure. In brief, the CM5 matrix was activated by injection of 70 μl of a 1:1 mixture of 400 mM N-ethyl-N′-(3-dimethyl aminopropyl)-carbodiimide hydrochloride and 100 mM N-hydroxysuccinimide at a flow rate of 20 μl min−1, followed by application of the appropriate recombinant protein solution. Activated functional groups on the sensor chip not saturated by proteins were blocked with 1 M ethanolamine. Binding of human CTRP6 was stopped when the desired level of about 100 resonance units was reached. Next, the indicated analyte (CTRP6, factor H, Sigma; C3(H2O), provided by T. Fujita) was injected. C3(H2O) was generated by five freeze/thawing cycles of C3 (ref. 62). Regeneration of the sensor chip was achieved by injecting the buffer described below for 3 min. The SPR assay and kinetics analysis was performed using a BIAcore 2000. The experiments were routinely performed in 10 mM HEPES (pH 7.4) buffer containing 150 mM NaCl, 3 mM EDTA and 0.005% surfactant P20 at a flow rate of 20 μl min−1.
Reverse passive Arthus (RPA) reaction
For IgG-mediated cutaneous RPA reaction, mice were intradermally injected with 10 μg anti-OVA Ab (Millipore, AB1225), and then intravenously with 500 μg OVA/1% Evans Blue (OVA; Sigma and Evans Blue; Wako) in PBS (pH 7.4). Skin was harvested 4 h later and incubated with dimethylformamide; eluted Evans blue in the supernatants was quantified by measuring the absorbance at 570 nm (ref. 63).
Experimental autoimmune encephalomyelitis (EAE)
The MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) was synthesized and purified by HPLC at our institute (Dr S. Imajoh-Ohmi, Division of Medical Proteomics Laboratory, Institute of Medical Science, University of Tokyo). Mice were immunized subcutaneously in flanks on day 0 with 300 μg MOG35–55 peptide emulsified in CFA (1:1), which consisted of IFA with 5 mg ml−1 M. tuberculosis (Difco). On day 7, mice were given a booster injection subcutaneously in flanks with the same amount of MOG/CFA. We judged the development of the severity of EAE by macroscopic evaluation. The severity score was graded as follows: 0, no change; 0.5, partially limp tail; 1, paralysed tail; 2, hind limp paresis; 2.5, one hindlimb paralysed; 3, both hindlimbs paralysed; 3.5, hindlimbs paralysed and weakness in forelimbs; 4, forelimbs paralysed64.
Gel filtration chromatography
C57BL/6 mouse serum (100 μl) or 1 μg/100 μl rhCTRP6 (Aviscera Bioscience) were fractionated through a 16/60 Sephacryl S-300 (GE Healthcare, USA) column (1.6 × 60 cm) in PBS at flow rate of 0.5 ml min−1. The fractionated samples were subjected to ELISA and the protein concentration was measured with a spectrophotometer. Thyroglobulin (669 kDa, Sigma), catalase (232 kDa, Sigma), BSA (66 kDa, Sigma) and OVA (45 kDa, Sigma) were used as molecular-weight makers.
Primary fibroblast-like synoviocytes
The primary synoviocytes were harvested from synovium of the knee and ankle and were cultured in DMEM medium (Gibco, USA) containing 10% fetal bovine serum and 1% penicillin–streptomycin. Cells were stimulated with IL-1α (0, 0.1, 1 and 10 ng ml−1) for 24 h. C1qtnf6 expression was measured by semi-quantitative PCR analysis.
BM-derived macrophages
To generate BM-derived macrophages, BM cells at 2 × 105 cells per millilitre were cultured in a 100-mm untreated dish using RPMI 1640 medium supplemented with 10% fetal bovine serum and 20 ng ml−1 recombinant macrophage colony-stimulating factor (PeproTech, USA). On day 3, we added another 10 ml of this medium to the dish. At day 7, we collected BM-derived macrophages and the cells at 1 × 105 cells per millilitre in a 96-well dish were stimulated with rhCTRP6 for 24 h. IL-10 levels in the culture supernatants were measured using Mouse IL-10 ELISA MAX Standard (Biolegend).
Samples from RA patients and healthy controls
Serum samples were collected from 30 Japanese RA patients diagnosed by rheumatologists according to the criteria of the American College of Rheumatology (ACR) in 1987 (ref. 65) or the 2010 ACR/EULAR classification criteria66. The mean age of the RA patients was 56.6 years; 20.0% were males. Serum samples were also collected from 22 healthy control subjects (mean age, 27.8 years; 18.2%, males). Frozen sections of synovium were fixed in 4% paraformaldehyde at room temperature for 15 min and blocked with 1% BSA in PBS for 30 min. For immunostaining of CTRP6, a rabbit anti-CTRP6 antibody (Abcam, ab36900, 1:100 dilution) and anti-rabbit IgG (Bio-Rad, 172–1019, 1:50 dilution) were used, and a rabbit IgG (Dako, Denmark, X0903, 1:100 dilution) was used as a control. Antibody binding was visualized using diaminobenzidine tetrahydrochloride (Nichirei Bioscience, Japan). For fluorescent immunostaining, a rabbit anti-CTRP6 antibody (Abcam, ab36900, 1:100 dilution), rabbit IgG (Bio-Rad, 172–1019, 1:50 dilution), mouse anti-CD3 antibody (Biolegend, 344802, 1:50 dilution), mouse anti-CD68 antibody (Biolegend, 556059, 1:50 dilution), mouse anti-Hsp47 antibody (Abcam, ab77609, 1:50 dilution), mouse IgG2b (BD Pharmingen, 557351, 1:50 dilution), mouse anti-CD20 antibody (Biolegend, 302301, 1:50 dilution), mouse IgG1 (Biolegend, 401401, 1:50 dilution), Alexa Fluor 488 anti-rabbit IgG (Invitrogen, A11034, 1:50 dilution) and Alexa Fluor 546 anti-mouse IgG (Invitrogen, A11030, 1:50 dilution) were used. Nuclei were stained with 4′,6-diamidino-2-phenylindole (Vector Labs, USA). The slides were visualized on a fluorescence microscope (Keyence, Japan). All the samples were collected from patients from whom we obtained informed consent at the University of Tsukuba Hospital. This study was reviewed and approved by the ethics committee of the University of Tsukuba.
Statistical analysis
Incidence of CIA, CAIA and EAE was evaluated by the χ2-test, and the severity score by the Mann–Whitney U-test. Student’s t-test was used for all other statistical evaluations.
Additional information
How to cite this article: Murayama, M. A. et al. CTRP6 is an endogenous complement regulator that can effectively treat induced arthritis. Nat. Commun. 6:8483 doi: 10.1038/ncomms9483 (2015).
References
Ricklin, D., Hajishengallis, G., Yang, K. & Lambris, J. D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 (2010).
Okroj, M., Heinegard, D., Holmdahl, R. & Blom, A. M. Rheumatoid arthritis and the complement system. Ann. Med. 39, 517–530 (2007).
Moxley, G. & Ruddy, S. Elevated C3 anaphylatoxin levels in synovial fluids from patients with rheumatoid arthritis. Arthritis Rheum. 28, 1089–1095 (1985).
Wang, Y. et al. A role for complement in antibody-mediated inflammation: C5-deficient DBA/1 mice are resistant to collagen-induced arthritis. J. Immunol. 164, 4340–4347 (2000).
Ji, H. et al. Arthritis critically dependent on innate immune system players. Immunity 16, 157–168 (2002).
Banda, N. K., Takahashi, K., Wood, A. K., Holers, V. M. & Arend, W. P. Pathogenic complement activation in collagen antibody-induced arthritis in mice requires amplification by the alternative pathway. J. Immunol. 179, 4101–4109 (2007).
Hashimoto, M. et al. Complement drives Th17 cell differentiation and triggers autoimmune arthritis. J. Exp. Med. 207, 1135–1143 (2010).
Ingram, G., Hakobyan, S., Robertson, N. P. & Morgan, B. P. Complement in multiple sclerosis: its role in disease and potential as a biomarker. Clin. Exp. Immunol. 155, 128–139 (2009).
Pickering, M. C. et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat. Genet. 31, 424–428 (2002).
Rose, K. L. et al. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J. Clin. Invest. 118, 608–618 (2008).
Dunkelberger, J. R. & Song, W. C. Complement and its role in innate and adaptive immune responses. Cell Res. 20, 34–50 (2010).
Feldmann, M. & Maini, S. R. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol. Rev. 223, 7–19 (2008).
Kishimoto, T. IL-6: from its discovery to clinical applications. Int. Immunol. 22, 347–352 (2010).
Edwards, J. C. & Cambridge, G. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology (Oxford) 40, 205–211 (2001).
Iwakura, Y. et al. Induction of inflammatory arthropathy resembling rheumatoid arthritis in mice transgenic for HTLV-I. Science 253, 1026–1028 (1991).
Horai, R. et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J. Exp. Med. 191, 313–320 (2000).
Fujikado, N., Saijo, S. & Iwakura, Y. Identification of arthritis-related gene clusters by microarray analysis of two independent mouse models for rheumatoid arthritis. Arthritis Res. Ther. 8, R100 (2006).
Wong, G. W. et al. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem. J. 416, 161–177 (2008).
Ghai, R. et al. C1q and its growing family. Immunobiology 212, 253–266 (2007).
Shapiro, L. & Scherer, P. E. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr. Biol. 8, 335–338 (1998).
Murayama, M. A. et al. CTRP3 plays an important role in the development of collagen-induced arthritis in mice. Biochem. Biophys. Res. Commun. 443, 42–48 (2014).
Trentham, D. E., Townes, A. S. & Kang, A. H. Autoimmunity to type II collagen an experimental model of arthritis. J. Exp. Med. 146, 857–868 (1977).
Komiyama, Y. et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177, 566–573 (2006).
Kohl, J. & Gessner, J. E. On the role of complement and Fc gamma-receptors in the Arthus reaction. Mol. Immunol. 36, 893–903 (1999).
Ogonuki, N. et al. A high-speed congenic strategy using first-wave male germ cells. PLoS ONE 4, e4943 (2009).
Niwa, H., Yamamura, K. & Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 (1991).
Wagner, E. & Frank, M. M. Therapeutic potential of complement modulation. Nat. Rev. Drug Discov. 9, 43–56 (2010).
Oran, A. E. & Isenman, D. E. Identification of residues within the 727-767 segment of human complement component C3 important for its interaction with factor H and with complement receptor 1 (CR1, CD35). J. Biol. Chem. 274, 5120–5130 (1999).
Peake, P. & Shen, Y. Factor H binds to the N-terminus of adiponectin and modulates complement activation. Biochem. Biophys. Res. Commun. 397, 361–366 (2010).
Nataf, S., Carroll, S. L., Wetsel, R. A., Szalai, A. J. & Barnum, S. R. Attenuation of experimental autoimmune demyelination in complement-deficient mice. J. Immunol. 165, 5867–5873 (2000).
Boos, L. A., Szalai, A. J. & Barnum, S. R. Murine complement C4 is not required for experimental autoimmune encephalomyelitis. Glia 49, 158–160 (2005).
Izquierdo, E. et al. Synovial fibroblast hyperplasia in rheumatoid arthritis: clinicopathologic correlations and partial reversal by anti-tumor necrosis factor therapy. Arthritis Rheum. 63, 2575–2583 (2011).
Mayilyan, K. R. Complement genetics, deficiencies, and disease associations. Protein Cell 3, 487–496 (2012).
Morgan, H. P. et al. Structural basis for engagement by complement factor H of C3b on a self surface. Nat. Struct. Mol. Biol. 18, 463–470 (2011).
Han, E. D., MacFarlane, R. C., Mulligan, A. N., Scafidi, J. & Davis, A. E. 3rd Increased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptor. J. Clin. Invest. 109, 1057–1063 (2002).
Xu, C. et al. A critical role for murine complement regulator crry in fetomaternal tolerance. Science 287, 498–501 (2000).
Oran, A. E. & Isenman, D. E. Identification of residues within the 727-767 segment of human complement component C3 important for its interaction with factor H and with complement receptor 1 (CR1, CD35). J. Biol. Chem. 274, 5120–5130 (1999).
Janssen, B. J. C. & Gros, P. Structural insights into the central complement component C3. Mol. Immunol. 44, 3–10 (2007).
Hietala, M. A., Jonsson, I. M., Tarkowski, A., Kleinau, S. & Pekna, M. Complement deficiency ameliorates collagen-induced arthritis in mice. J. Immunol. 169, 454–459 (2002).
Arnold, J. N., Wormald, M. R., Sim, R. B., Rudd, P. M. & Dwek, R. A. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 25, 21–50 (2007).
Endo, T., Iwakura, Y. & Kobata, A. Structural changes in the N-linked sugar chains of serum immunoglobulin G of HTLV-I transgenic mice. Biochem. Biophys. Res. Commun. 192, 1004–1010 (1993).
Banda, N. K. et al. Initiation of the alternative pathway of murine complement by immune complexes is dependent on N-glycans in IgG antibodies. Arthritis Rheum. 58, 3081–3089 (2008).
Iwakura, Y., Ishigame, H., Saijo, S. & Nakae, S. Functional specialization of interleukin-17 family members. Immunity 34, 149–162 (2011).
Strainic, M. G., Shevach, E. M., An, F., Lin, F. & Medof, M. E. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat. Immunol. 14, 162–171 (2013).
Kim, M. J., Lee, W., Park, E. J. & Park, S. Y. C1qTNF-related protein-6 increases the expression of interleukin-10 in macrophages. Mol. Cells 30, 59–64 (2010).
Keystone, E., Wherry, J. & Grint, P. IL-10 as a therapeutic strategy in the treatment of rheumatoid arthritis. Rheum. Dis. Clin. North Am. 24, 629–639 (1998).
Seldin, M. M., Tan, S. Y. & Wong, G. W. Metabolic function of the CTRP family of hormones. Rev. Endocr. Metab. Disord. 15, 111–123 (2014).
Woodruff, T. M., Nandakumar, K. S. & Tedesco, F. Inhibiting the C5-C5a receptor axis. Mol. Immunol. 48, 1631–1642 (2011).
Julia, A. et al. Genome-wide association study of rheumatoid arthritis in the Spanish population: KLF12 as a risk locus for rheumatoid arthritis susceptibility. Arthritis Rheum. 58, 2275–2286 (2008).
Barrett, J. C. et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 41, 703–707 (2009).
Jin, Y. et al. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N. Engl. J. Med. 362, 1686–1697 (2010).
Cooper, J. D. et al. Meta-analysis of genome-wide association study data identifies additional type 1 diabetes risk loci. Nat. Genet. 40, 1399–1401 (2008).
Atkinson, J. P. & Goodship, T. H. Complement factor H and the hemolytic uremic syndrome. J. Exp. Med. 204, 1245–1248 (2007).
Nagy, A., Rossant, J., Nagy, R., Abramow-Newerly, W. & Roder, J. C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl Acad. Sci. USA 90, 8424–8428 (1993).
Horai, R. et al. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J. Exp. Med. 187, 1463–1475 (1998).
Inglis, J. J., Simelyte, E., McCann, F. E., Criado, G. & Williams, R. O. Protocol for the induction of arthritis in C57BL/6 mice. Nat. Protoc. 3, 612–618 (2008).
Brand, D. D., Latham, K. A. & Rosloniec, E. F. Collagen-induced arthritis. Nat. Protoc. 2, 1269–1275 (2007).
Khachigian, L. M. Collagen antibody-induced arthritis. Nat. Protoc. 1, 2512–2516 (2006).
Lachmann, P. J. Preparing serum for functional complement assays. J. Immunol. Methods 352, 195–197 (2010).
Seelen, M. A. et al. Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J. Immunol. Methods 296, 187–198 (2005).
Kimura, Y., Miwa, T., Zhou, L. & Song, W. C. Activator-specific requirement of properdin in the initiation and amplification of the alternative pathway complement. Blood 111, 732–740 (2008).
McRae, J. L. et al. Human factor H-related protein 5 has cofactor activity, inhibits C3 convertase activity, binds heparin and C-reactive protein, and associates with lipoprotein. J. Immunol. 174, 6250–6256 (2005).
Utomo, A. et al. Requirement for Vav proteins in post-recruitment neutrophil cytotoxicity in IgG but not complement C3-dependent injury. J. Immunol. 180, 6279–6287 (2008).
Stromnes, I. M. & Goverman, J. M. Active induction of experimental allergic encephalomyelitis. Nat. Protoc. 1, 1810–1819 (2006).
Arnett, F. C. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31, 315–324 (1988).
Aletaha, D. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum. 62, 2569–2581 (2010).
Acknowledgements
We thank Dr Yuko Kimura and Dr Wen-Chao Song for assistance with the complement activation assay and Dr Junichi Miyazaki for the pCXN2 vector. We thank Dr Masato Nose for his excellent histological analysis of arthritis and thoughtful discussion. We thank Dr Shuhei Ogawa for an installation of C3−/− mice, and all the members of our laboratory for kind cooperation, discussion and animal care. This work was supported by CREST of the Japan Science and Technology Agency (to Y.I.), the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry (to Y.I.), Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (to Y.I.) and M.A.M. is a research assistant of the of the CREST program.
Author information
Authors and Affiliations
Contributions
M.A.M. was the primary contributor to this work and wrote the manuscript. S.K. and S.S. gave advice and technical support. A.I., N.U. and T.S. performed all experiments using human samples. T.Y. and R.K. carried out western blotting and SPR-based interaction assays. H.I. generated the targeting construct for generation of C1qtnf6−/− mice, and T.T., S.K., N.S. and Y.L. generated KO and Tg mice. R.Y., S.I., T.M. and A.S. assisted with gel filtration chromatography, flow cytometric analysis, immunohistochemistry and EAE, respectively. H.-H.C. and Y.H. helped with qPCR analysis. K.T. and M.M. performed C3 convertase activity assays. T.F. provided recombinant complement components and M.H. gave advice. Y.I. organized and supervised the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-10, Supplementary Tables 1-3, Supplementary Methods and Supplementary References (PDF 1992 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Murayama, M., Kakuta, S., Inoue, A. et al. CTRP6 is an endogenous complement regulator that can effectively treat induced arthritis. Nat Commun 6, 8483 (2015). https://doi.org/10.1038/ncomms9483
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms9483
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.