Abstract
T-cell acute lymphoblastic leukaemias (T-ALL) are aggressive malignant proliferations characterized by high relapse rates and great genetic heterogeneity. TAL1 is amongst the most frequently deregulated oncogenes. Yet, over half of the TAL1+ cases lack TAL1 lesions, suggesting unrecognized (epi)genetic deregulation mechanisms. Here we show that TAL1 is normally silenced in the T-cell lineage, and that the polycomb H3K27me3-repressive mark is focally diminished in TAL1+ T-ALLs. Sequencing reveals that >20% of monoallelic TAL1+ patients without previously known alterations display microinsertions or RAG1/2-mediated episomal reintegration in a single site 5′ to TAL1. Using ‘allelic-ChIP’ and CrispR assays, we demonstrate that such insertions induce a selective switch from H3K27me3 to H3K27ac at the inserted but not the germline allele. We also show that, despite a considerable mechanistic diversity, the mode of oncogenic TAL1 activation, rather than expression levels, impact on clinical outcome. Altogether, these studies establish site-specific epigenetic desilencing as a mechanism of oncogenic activation.
Similar content being viewed by others
Introduction
T-cell acute lymphoblastic leukaemia’s (T-ALL) are malignant proliferations of immature T-cell progenitors. Although the outcome of T-ALLs has greatly improved in the last 10 years, ~30% of cases relapse within the first 2 years following diagnosis; moreover, acute- and long-term toxicities remain important issues for long-term survivors, underlining the critical need of better risk stratification of T-ALL patients, and the implementation of more adapted and/or targeted therapies. A major obstacle in the molecular dissection of these processes is that T-ALLs constitute a particularly heterogeneous group of disease, characterized by complex combinations of multigenic aberrations and oncogenic cooperation. The deregulation of over 40 distinct oncogenes and tumour suppressors has been reported, occurring through a large diversity of genomic aberrations and epigenetic mechanisms1,2. Chromosomal alterations mostly consist of translocations, inversions and microdeletions occurring at the vicinity of proto-oncogenes and leading to their deregulated expression.
TAL1 is one of the most frequently deregulated T-ALL oncogenes3. In physiological conditions, TAL1 is a regulatory gene that promotes access to alternative fates in haematopoiesis. Expressed in early haematopoiesis, its expression is maintained in the erythroid lineage, but normally irreversibly epigenetically silenced in the T-cell lineage4,5,6 (Supplementary Fig. 1). Events leading to the illegitimate maintenance or re-expression of TAL1 in the T-cell lineage are thought to constitute strong drivers of T-ALL leukemogenesis. Known TAL1 dysregulation mechanisms consist of t(1;14)(p34;q11) translocations (1–2%) and SIL-TAL deletions (15–20%). Yet, over half of the TAL1+ cases lack TAL1 lesions, suggesting unrecognized (epi)genetic deregulation pathways7. In such ‘unresolved cases’, TAL1 expression can be monoallelic, compatible with a direct alteration in cis within or around the TAL1 gene, or biallelic, likely reflecting indirect deregulation in trans7.
Here we sought to gain insights into the (epi)genetic deregulation pathways of TAL1 ‘unresolved’ cases. Using ChIP-seq and an ‘allelic-ChIP’ assay allowing to discriminate histone marks separately on each allele, we describe a new process of oncogene activation, whereby the targeted eviction of polycomb H3K27me3 marks and concurrent recruitment of H3K27ac marks by micro- and macroinsertional events, trigger the monoallelic desilencing of TAL1. Incidentally, we also report the first example of oncogenic activation by recombination-activating gene (RAG)-mediated episomal reinsertion, a very elusive event predicted in vitro as a source of oncogenic activation over 10 years ago8,9, but never identified before in human cancer. We further show that this new epigenetic desilencing process is a recurrent event in T-ALL, accounting for >20% of unexplained cases of monoallelic TAL1 oncogene activation. Finally, we demonstrate that the mode of activation (monoallelic in cis versus biallelic in trans) rather than the level of TAL1 expression impacts on prognosis, with cis-mediated alterations significantly associated with adverse clinical outcome.
Results
Microinsertions induce epigenetic switch and TAL1 expression
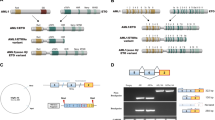
To investigate the (epi)genetic deregulation pathways of TAL1 ‘unresolved’ cases, we first took advantage of chromatin immunoprecipitation coupled to a high-throughput sequencing (ChIP-seq) data set describing active/inactive epigenetic marks in mouse developing thymocytes6,10. A large region starting upstream of the TAL1 promoter and encompassing part of the gene body was enriched in H3K27me3, suggesting the involvement of polycomb complex (PcG) repressive activity in TAL1 silencing (Fig. 1a). This profile was also observed in human TAL1− peripheral CD4+ T cells11, but absent in TAL1-expressing cells12 (CD34+ haematopoietic stem cells, HSC; erythroblasts; Fig. 1b), in line with the gradual deposition of H3K27me3-repressive marks during T-cell lineage specification6 (Supplementary Fig. 1). To determine if deregulation of this silencing occurs in TAL1+ T-ALLs, ChIP-seq was also performed in the Jurkat (TAL1+) T-ALL cell-line and a control TAL1− sample (DA). Sample DA displayed the expected H3K27me3 deposition, coherent with TAL1 silencing in T cells (Fig. 1b). Surprisingly, however, low levels of H3K27me3 marks were also observed in Jurkat. Since, in contrast to normal TAL1-expressing cells, Jurkat displays monoallelic expression of TAL1 (Supplementary Fig. 2), we reasoned that a monoallelic chromosomal alteration might have prevented H3K27me3 deposition on one of the two alleles, resulting in an averaged, intermediate ChIP-seq signal. To test this possibility, we sequenced ~10 kb upstream of TAL1 (Supplementary Fig. 3), covering the regulatory region enriched in H3K27me3 marks. Amid single-nucleotide polymorphisms (SNPs), we found a monoallelic alteration consisting of a 12-bp microinsertion ~7 kb upstream of the TAL1 p1a promoter, in a region prone to DNA looping with TAL1 promoters13. Interestingly, this insertion mapped at the border of the repressive H3K27me3 pattern (Fig. 1a,b), in line with the possibility that it disrupted normal epigenetic silencing of the TAL1 locus. To further investigate the association of the insertion with potential allelic distortion of the repressive H3K27me3 mark, we performed an ‘allelic-ChIP’ that discriminates H3K27me3 marks at the inserted versus germline allele (Fig. 2a). A significant decrease of H3K27me3 signal was observed at the inserted allele compared with the germline allele (P<0.005). Accordingly, knockdown (by shEZH2) or inhibition (using the EZH2 inhibitor GSK126) (ref. 14) of the Polycomb H3K27me3 methyl transferase EZH2, allowed partial reversion of H3K27me3 deposition at the germline (non-inserted) allele. Considering the cooperative role of histone modifications in expression regulation, we further analysed the relevance of acetylation in presence or absence of histone deacetylase inhibitor (sodium butyrate)15. ChIP-seq showed a slight enrichment of H3K27ac with significant overall increase on sodium butyrate treatment (Fig. 2b). Similar to H3K27me3, H3K27ac marks extended to the insertion site, suggesting dual epigenetic regulation and differential allelic recruitment. Indeed, allelic quantification through tag retrieval and allelic-ChIP revealed a difference of H3K27ac between inserted and GL alleles in a pattern symmetrical to and functionally coherent with H3K27me3. Acetylation levels were histone deacetylase inhibitor dependent. Overall, this suggested that Jurkat’s microinsertion contributed to site- and allele-specific switch from H3K27me3 to H3K27ac deposition, leading to the maintenance of TAL1 expression through T-cell differentiation.
(a) ChIP-seq profiles of chromatin marks in normal mouse CD4+CD8+ thymocytes. The TAL1 genomic area on chromosome 4 (including the adjacent STIL gene, which is physiologically expressed at that developmental stage) is shown for TATA-binding protein-GTF recruited to promoters (TBP), Polymerase II (Pol II), H3K4me1 (active chromatin mark for enhancer/regulatory regions), H3K4me3 (active chromatin marks for promoters), H3K36me3 (active chromatin marks for gene bodies) and H3K27me3 (repressive chromatin mark dependent on the PcG). The TAL1 region is zoomed, and the insertion breakpoint localization to the human orthologue region (114,722,607) is indicated by a red arrow. (b) ChIP-seq profiles of polycomb repressive chromatin mark H3K27me3 in normal human TAL1+ (HSC; erythroblasts) and TAL1− (peripheral CD4+ T cells) lineages, compared with TAL1+ (Jurkat) and TAL1− (DA) T-ALL cells. The TAL1 gene and surrounding area on chromosome 1 (including the STIL gene) is shown. The insertion breakpoint localization (47704964, HG19 coordinates) is indicated by an arrow. All profiles were input subtracted, except for HSC and erythroblasts for which input data were not available12. Significantly enriched areas are represented as green rectangles under the lanes (MACS2 peaks).
(a) Allelic-ChIP assay of H3K27me3 marks. Top panel: the assay to discriminate the germline (GL) from the inserted allele (Ins.) by substituting one of the GL primers allowing detection of the GL configuration at the insertion site (Rev. TAL1), with an insertion-specific primer allowing detection of the inserted configuration (Rev. Ins, overlapping the 12-bp insertion). Primer pairs were tested on GL p(GL) or inserted p(Ins) cloned fragments (and on cell lines containing (Jurkat) or not (DND) the insertion) to exclusively amplify each configuration, and do not crossreact. Bottom middle panel: western blot of EZH2 protein content on shMock or EZH2 knockdown conditions. Allelic-ChIP assays were performed in presence of a non-silencing sh-RNA (shControl) or a sh-RNA-targeting EZH2 (shEZH2) (left panel) or after the incubation of Jurkat cells with GSK126 (0.5 μM, 72H) or vehicle (dimethyl sulphoxide, DMSO; right panel). GAPDH and HoxD11 were used as controls for activated/repressed genes, modulated according to the polycomb-dependent H3K27me3 marks. Note that EZH2 knockdown/inhibition triggered only partial decrease of H3K27me3 marks at the PcG-repressed HoxD11 control gene, possibly due to incomplete knockdown/inhibition and/or redundancy of polycomb components in the adult lymphoid lineage42. (b) Enrichment of acetylation marks at the TAL1 locus. H3K27Ac ChIP was performed with Jurkat cells incubated with vehicle (DMSO) or the histone deacetylase inhibitor sodium butyrate (SOB) (5 mM, 4H); DNA was then analysed by ChIP-seq (left panel) or by allelic-ChIP (bottom panel). For the ChIP-Seq, quantification of the number of tag sequences at the insertion point is shown (right panel). ***P<0.001; **P<0.001; *P<0.05, unpaired t-test. Errors bars represent 95% confidence interval.
A recurrent epigenetic mechanism of TAL1 deregulation
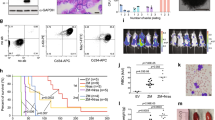
To determine if similar structural abnormalities occurred recurrently in TAL1 patients, 134 primary T-ALL samples were analysed by high-density Affymetrix SNP array-6 analysis; an ~700-bp region surrounding the Jurkat insertion site was also sequenced in a subset of 93 samples and six cell lines; in parallel, the literature was reviewed for cases with unexplained TAL1 activation. While no macromolecular TAL1 alteration was identified by SNP array (0/134), sequencing revealed seven new cases of similar microinsertions (1–9 bp), all precisely located at the Jurkat insertion breakpoint (Fig. 3a). Such insertions were not present in the germline from 2/2 available patients tested (Supplementary Fig. 4). No additional mutation/indel could be found in the surrounding ~700 bp region in tumour samples. Significantly, insertions were exclusively found in TAL1+ patients; moreover, among patients with informative SNPs in the TAL1 3′ UTR allowing distinction of mono- from biallelic expression7 (n=60), insertions were exclusively found in monoallelic cases (4/19, >20%), in agreement with a cis-mode of TAL1 activation (Fig. 3b).
(a) Nucleotide sequences of episomal/microinsertions. All insertions were specifically and exclusively located at the indicated genomic position, and are pictured in red. Nucleotide deletions are indicated by a red dash. No SNPs are referenced at this position (Supplementary Fig. 8); (b) Relative TAL1 expression in T-ALL patients (n=111) according to biallelic (BI), or monoallelic (MONO) expression; patients with micro/episomal insertions are indicated in red; SIL-TAL cases are shown separately; informative cases: the presence of SNPs in TAL1 3’UTR allows to determine if the expression is mono- or biallelic. NI: non-informative cases (absence of SNPs in TAL1 3′ UTR does not allow to determine if the expression is mono- or biallelic). The average physiological TAL1 levels in thymus is shown as reference (Thymus); Horizontal bars indicate median expression levels; **indicates significant difference between BI and MONO expression (Mann–Whitney U-test, P<0,01); note that a number of biallelic patients are reaching/below physiological thymus levels, and might result from the presence of residual TAL1-expressing erythroblasts among tumoral cells43; TAL1 expression was analyzed by Taqman assay and is normalized to ABL (see Methods).
Oncogenic RAG1/2-mediated episomal reintegration
One additional candidate was recovered by data mining16. In this case (patient #OC), TAL1high activation concurred with the insertion of a large piece of chromosome 7 disrupting the TAL1 locus. Strikingly, breakpoint mapping by ligation-mediated PCR revealed that the insertion occurred at the very same insertion site, although this time with few nucleotide deletions on each side of the breakpoint (Fig. 3a). Detailed analysis of the junctions revealed the occurrence of RAG1/2-mediated reinsertion of an ~370-kb TCRβ episomal circle (TRECβ, excised during normal V(D)J recombination, Fig. 4 and Supplementary Fig. 5). This establishes the first example of oncogenic RAG1/2-mediated reintegration, demonstrating that TRECs may indeed contribute to oncogenesis17. Owing to the large TRECβ size, we considered the possibility that, unlike other microinsertions, a promoter located in the episome could have initiated a >7-kb-long fusion transcript encompassing TAL1 (Supplementary Fig. 6). However, reverse transcription-PCR (RT–PCR) exon walking and 5′ RACE assays indicated that transcripts initiated from the TAL1 p4 promoter, excluding this possibility.
The TCRβ locus is displayed (top lane, not to scale). A functional Vβ7.4-to-Dβ1 rearrangement generating an excised TRECβ, and containing a (Vβ7.4/Dβ1) signal joint (SJ) is represented. The episome might have been open at the SJ by a nick–nick process44 generating 3′ hydroxyl ends before integration in chromosome 1. The episome is integrated in reverse orientation 10 kb downstream of the STIL gene, and 7 kb upstream of the TAL1 gene (middle lane). A 10-bp deletion (Δ, underlined) occurred at the insertion site. Localization of cryptic RSSs used by illegitimate V(D)J-mediated SIL-TAL deletion, and by t(1;14) TCRδ/TAL1 translocations are indicated by black arrow heads. TAL1 promoters (P1a, P1b, P4) are indicated. The breakpoints sequences (Bkp1/2) are shown (bottom lane). N, N regions; Vβ7.4 and Dβ1 RSSs are indicated, with heptamers (7) and nonamers (9) depicted.
Epigenetic modulation and TAL1 gene expression
Allelic-ChIP was then performed on patients #TAMFA and #OC. Similar to Jurkat, significant enrichment of the repressive H3K27me3 mark was consistently observed in germline compared with inserted alleles (Fig. 5a,b). The amplitude of the allelic distortion appeared higher in OC than in Jurkat and TAMFA, possibly due to the large difference in the insertion size. Of note, TAL1 transcription levels were also higher in OC (Fig. 3b). Using CrispR DNA editing, we next mimicked site-specific insertion and disruption of the region 7 kb 5′ of TAL1 in the TAL-negative PEER cell line (Fig. 5b). In clone #2.4 recapitulating the 12-bp Jurkat insertion at its 3′ end, an approximately fivefold increase of TAL1 could be observed. While we cannot formally exclude the possibility that the selection cassette contributed to the fivefold change in clone #2.4, a 55-fold increase was observed in clone #5.10, in which an ~1.3 kb deletion 5′ of the insertion site mimicked locus disruption in patient #OC; furthermore, this was accompanied by an allelic switch from H3K27 methylation to acetylation. This provides direct evidence for a causal relationship between site mutagenesis, epigenetic modulation and TAL1 gene expression.
(a) Allelic-ChIP analysis of H3K27me3 marks at the insertion breakpoint in primary patients. See legend to Fig. 2a. Marks at the GL alleles in one TAL1+ biallelic (RENE) and in one TAL1− (DAV) patients were performed as controls. ***P<0.001, unpaired t-test; Relative-fold plots: Inserted and/or GL allele ChIP values were calculated as fold increases relative to GAPDH (numbers in blue) or HoxD11 (numbers in red), and folds plotted as relative percentages (GAPDH relative folds: blue histograms; HOXD11 relative folds: red histograms). Blue histograms over 50% indicate higher differences with the expressed than the repressed control genes and correspond to (partially) repressed TAL1 expression; conversely, red histograms over 50% indicate higher differences with the repressed than the expressed control genes and correspond to (partially) derepressed TAL1 expression; histograms are ordered according to decreasing TAL1 repression. (b) Epigenetic modulation and TAL1 gene expression by DNA editing mimicking insertional mutagenesis. Left panel: schematic representation of the CRISPR design for homologous recombination at the TAL1 locus, and configuration of two edited clones in the PEER cell line. The locations of PCR primers (plain arrows) for detecting successful targeted events and for genome walking are indicated. Bottom left panel: successful homologous recombination was confirmed by PCR of the expected genome-donor and donor-insert boundaries. Top right panel: RQ-PCR analysis of TAL1 expression after editing. Transcripts were normalized to ABL and reported as relative values to non-edited PEER cells. Four PCR replicates were performed on 1 (clone 2.4, due to impaired growth) or 2 (clone 5.10) independent RNA extractions. Bottom right panel: allelic-ChIP assays of H3K27me3 and H3K27ac marks in edited clone 5.10. See legend to Fig. 2a. ***P<0.001, unpaired t-test.
The mode of TAL1 activation impacts on clinical outcome
Patients with identified insertions were globally of adverse prognosis. We sought to determine if clinical outcome correlated with quantitative or qualitative aspects of TAL1 deregulation. A cohort of 165 adult T-ALL treated prospectively in the GRAALL (Group for Research in Adult Acute Lymphoblastic Leukaemia) trial was split into TAL1 expression quartiles, and compared for disease-free (DFS) and overall survival (OS). The seven patients with identified insertions (three of whom were GRAALL treated), all belonged to the high-expression quartiles (Q3–4). However, no significant difference in survival was observed between the quartiles (Fig. 6a,b), suggesting that quantitative TAL1 expression does not correlate with clinical outcome. We next tested whether cis-mediated TAL1 alterations leading to monoallelic expression (including or not the SIL-TAL1+ cases) affected the clinical outcome compared with trans-mediated events, associated with biallelic TAL1 expression. Clinical outcome was indeed found to be significantly improved in the biallelic group (DFS, P=0.04; OS, P=0.03; Fig. 6c–f). Although numbers are low, monoallelic cases retained an inferior OS trend in multivariate analysis (including age, leukocytosis; P=0.07, Cox analysis). Despite genetic heterogeneity, monoallelic cases also displayed higher blasts counts at diagnosis than biallelic cases and a significantly lower frequency of deregulation of recurrent oncogenes such as TLX1, CALM-AF10 and TLX3 (Supplementary Table 1). This supports the emerging notion that the mode of alteration may shape the oncogenic landscape in a more profound manner than (and potentially override the effect of) transcriptional levels, and that this may eventually impact on the tumour’s clinical behaviour18. Deciphering the mechanisms underlying the (epi)genetic deregulation of biallelic TAL1+ cases will be instrumental to resolving this issue19,20,21,22 (Supplementary Fig. 7 and Supplementary Table 2).
Discussion
Establishing the detailed maps of the complex oncogenic networks involved in T-ALL has contributed to major genetic discoveries, and has been of prime importance for further therapeutic improvement. Over three decades of intense efforts in genomic research have allowed unravelling the extraordinary diversity of the mechanisms by which oncogenes are deregulated in this disease1. Yet, a large number of major oncogene deregulations still remain unexplained to date. Among the diversity of mechanisms involved, V(D)J recombination-mediated alterations (translocations, microdeletions) constitute the hallmarks of T-ALLs23,24,25,26. Interestingly, despite arrays of biochemical and functional evidence that the reintegration of excised episomal circles (TRECs) by the V(D)J recombinase (RAG1/2) might constitute a potent source of genomic instability, such events remained so far unreported in human cancer patients8,9,27,28,29,30. Here we report the first case of such an oncogenic RAG1/2-mediated episomal reintegration, demonstrating that TRECs can indeed contribute to human oncogenesis (Supplementary Fig. 1). Most intriguingly, this insertion occurred in a pediatric SCID-X1 patient who developed a leukaemia secondary to retroviral reinsertion (in front of LMO2, a known TAL1-cooperating oncogene) following gene therapy16. The screening of two other SCID-X1 patients’ leukaemic samples31,32, and of a large collection of T-ALLs did not reveal additional episomal insertions, and the reason for the extraordinary coincidence of two rare oncogenic integration events in this patient remains unanswered.
This and the other insertional mutagenesis T-ALL cases described here also revealed a novel oncogenic activation pathway, whereby a genetic alteration drives a site-specific and monoallelic epigenetic deregulation. We demonstrate that such insertions drove a switch from H3K27me3 to H3K27ac deposition, leading to the maintenance and/or re-expression of TAL1 expression through T-cell differentiation. Interestingly, the difference in TAL1 expression levels observed in mutants from the gene editing assay (Fig. 5) suggest that while small insertions might be sufficient to prevent the deposition of PcG repressive marks during T-cell lineage specification (thus permitting H3K27ac switch and maintenance of TAL1 expression), further disruption of the region 5′ of the insertion site (by deletion or insertional uncoupling) might be necessary to impose desilencing once TAL1 extinction is established in the T-cell lineage6 (Supplementary Fig. 1). These kinetics are coherent with thymocyte ontogeny in patient #OC, in which TRECβ rearrangement/reintegration (DN2-3) likely occurred after TAL1 silencing (DN1-2). Altogether, our data are in line with current models of permanent gene extinction of transcription factors during T-lineage commitment6 (Supplementary Fig. 1) and further identify locus control regions involved in deposition and/or maintenance of TAL1 silencing. Their genetic disruption constitute a recurrent epigenetic mechanism of TAL1 deregulation in T-ALLs, contributing to a substantial fraction (>20%) of the TAL1+ monoallelic ‘unresolved cases’, and associated with adverse prognostic. That a cis-deregulation regrouping as diverse mechanisms as SIL-TAL deletions, translocations or insertional desilencing impact more on prognosis than TAL1 expression levels underlines the fundamental oncogenic difference between a deregulation targeting a single locus, and the wider effect of trans-acting factors. Transcription factors indeed often bind to a large number of target genes (hundreds to thousands) and their deregulation (whether gain or loss) will likely affect a complex set of cellular functions, some of which might antagonize tumour progression, or resistance to treatment. Recently, reports identifying loss-of-function mutations in polycomb-related components19,20,21,33 have provided the framework by which global epigenetic modification might trigger the indirect (and biallelic) activation of numerous target genes, likely including a complex and conflicting set of oncogenes and tumour suppressors. In humans, PcG are recruited to and repress specific regions in the genome through as yet undefined set(s) of DNA-binding transcription factors and long non-coding RNAs34. The insertional mutagenesis described here identifies a site- and allele-specific switch from H3K27me3 recruitment/maintenance to H3K27ac, providing new avenues to decipher the mechanisms and DNA-binding intermediates involved3. A complex interplay between transcription factors and PcG would be in line with recent findings that Notch1 activation antagonizes PRC2 silencing of Notch1 target genes in T-ALL oncogenesis19. In intricate T-ALL networks where both NOTCH and TAL1 deregulation can coexist, we find that while NOTCH patients are associated with a favourable prognosis, monoallelic, but not biallelic TAL1, expression tends to convert the clinical outcome towards more adverse prognosis in the Notch subgroup, and to further aggravate the bad prognosis of Notch1WT patients. This suggests that distinct modes of deregulation of the same epigenetic complex might coexist in a tumour cell, leading to complex and potentially conflicting clinical outcomes which ought to be clarified when considering epigenetic inhibitors for new lines of treatment15,33,35.
Methods
ChIP
ChIP was performed as previously described10. In brief, the cells were chemically crosslinked by the addition of one-tenth volume of fresh 11% formaldehyde solution for 10 min at room temperature. Following the quenching of the reaction with glycine (250 mM final concentration, 5 min, room temperature), cells were rinsed twice with 1 × PBS and flash frozen in liquid nitrogen and stored at −80 °C before use. Cells were resuspended, lysed and sonicated to solubilize and shear crosslinked DNA. Sonication was conducted using a Bioruptor (Next Gen, Diagenode) for 15 min (30 s on, 30 s off), resulting in sheared DNA between 100 and 400 bp with the bulk at ~250 bp. The resulting whole-cell extract was incubated overnight at 4 °C with 100 μl of Dynal Protein G magnetic beads that had been preincubated with the appropriate antibody. The anti-H3K27me3 antibodies used were: # 07-449, Millipore (2 μg), and # ab6002, Abcam (1 μg); the anti-H3K27ac antibodies used were: #ab4729, Abcam (2 μg) and # 39133, active motif (5 μg). Beads were washed eight times with RIPA buffer and one time with TE containing 50 mM NaCl. Bound complexes were eluted from the beads by heating at 65 °C with occasional vortexing, and crosslinking was reversed by overnight incubation at 65 °C. Whole-cell extract DNA (reserved from the sonication step) was also treated for crosslink reversal. Immunoprecipitated DNA and whole-cell extract DNA were then purified by treatment with RNaseA, proteinase K and multiple phenol:chloroform: isoamyl alcohol extractions.
ChIP-seq
Before sequencing, ChIP DNA was quantified using the picogreen method (Invitrogen, USA) and quality controlled on a 2100 Bioanalyzer (Agilent). At least 1 ng of ChIP or input DNA was used for library preparation according to the Illumina ChIP-seq protocol. After end repair and adapter ligation, fragments were size selected on a gel before preamplification and clustering. The resulting fragments were again verified on a 2100 Bioanalyzer before clustering and 25 or 36 cycle sequencing on a Genome Analyzer II (Illumina, USA) according to the manufacturer’s instructions. Raw data were bowtie aligned and the tags elongated and further processed to wiggle files as described36. ChIP-seq experiments were performed on two independent biological replicates (samples for primary cells or culture for cell lines). Data presented in Fig. 1 are available under accession number GSE29362 for TBP, Pol II, H3K4me1, H3K4me3 and H3K36me3 or GSE38577for H3K27me3(1A), and for H3K27me3 in human cells (1B) GSE12646 for HSC and erythroblast, GSE12889 for CD4+ and GSE59257 for the data newly generated (Jurkat and DA) in this article. Technical replicates were merged before alignment with Bowtie and the resulting BAM files were used as treatment in MACS2. Input data sets were used as control when available (Jurkat, DA). Enriched regions for genomic tracks of Fig. 1a,b were extracted as BED files using MACS2 peak detection algorithm with the following parameters : genome sizes=2.70e+09 for human and 1.87e+09 for mouse, bandwidth=300, model fold=(5, 50), q value cutoff=5.00e−02, larger scaled towards smaller, λ range=1,000–10,000 bps, Broad on.
Assessment of wt versus inserted ChIP-seq signal
To identify a difference in H3K27ac enrichment in ChIP-seq between alleles with and without insertion (12 bp) in Fig. 2b, we aligned all 25 bp reads of each experiment to this specific region of insertion using the R package ‘Biostrings’. This alignment was performed against a region of 52 bp around the insertion site allowing for up to two mismatches, and the number of hits on forward and reverse strands was cumulated. For the mutant allele, the Jurkat insert sequence (5′-CCGTTTCCTAAG-3′) was inserted in the reference sequence before alignment, extending the initial alignment region to a size of 64 bp.
Allelic-ChIP
Input and IP genomic DNAs were analysed by RT–PCR using power SybrGreen on a 7,500 Fast Real-time PCR system (Applied Biosystems). IgG control ‘cycle over the threshold’ Ct values were subtracted to Input or IP Ct values and converted into bound value by 2−(IP Ct or input Ct- IgG IP Ct). Allelic-ChIP was carried out using allele-specific primers (by substituting one of the germline primers with a primer located in or overlapping the insertion). EZH2 knockdown was achieved using a doxycycline-induced short hairpin RNA (sh-RNA)-targeting EZH2 (pTRIPZ-EZH2, openbiosystem # V21HS-63066). A non-silencing sh-RNA (pTRIPZ-NS) was used as control. Jurkat cells were electroporated and cells containing the pTRIPZ were selected on puromycine. Knockdown of EZH2 was obtained by the addition of doxycycline (2 μg ml−1) to the cells during 10 days. Western blot was performed using the anti-EZH2 BD Biosciences # 612666.
Sequencing and SNP array
For Jurkat mapping, a region of 10 kb 5′ of TAL1 exon 1 was mapped on both alleles by LRPCR/cloning and standard Sanger sequencing as previously described37 (see Supplementary Fig. 2 for details on mapping strategy). Identification of allelic variants (SNPs versus somatic indels/mutations) was performed with vector NTI using alignment against reference TAL1 sequence and variants (http://www.ncbi.nlm.nih.gov/SNP; http://projects.tcag.ca/variation, Supplementary Fig. 8). For the sequencing screen on T-ALL patients and cell lines, a region of ~700 bp surrounding the Jurkat insertion site was directly PCR/sequenced on both strands in a subset of 93 samples and six cell lines. Heterogeneous sequences (ambiguous reading due to allelic differences) were systematically cloned, sequenced and analysed as above. For SNP array, hybridization on Affymetrix Cytogenetics SNP Array-6 was performed according to the manufacturer’s recommendations. Data analysis was performed with Chromosome Analysis Suite software using the following settings: the CGH log2 copy number ratio for heterozygous deletion was defined as 0.5 to 1.5, whereas log2 copy number ratios <1.5 were defined as homozygous deletions. Gene copy number (GCN) aberrations were compared with the Database of Genomic variants (http://projects.tcag.ca/variation) to study only non-variant GCV aberrations.
Patients
Diagnostic samples from a consecutive series T-ALLs from 165 adults (older than 16 years) included in GRAALL-03/05 trial (registration #NCT00327678 and #NCT00222027) were analysed for TAL1 expression. Sample collection and analyses were approved by the local ethical committee. Informed consent was obtained from the patients or relatives in accordance with the Declaration of Helsinki, with the institutional review board approval of all involved hospitals. Diagnosis of T-ALL was based on the World Health Organization 2008 criteria, defined by expression of cytoplasmic and/or surface CD3, and negativity of CD19 and MPO, as reported38. To be included, samples had to contain at least 80% of lymphoblasts. Immuno-geno/phenotyping and oncogene quantification were performed as previously described38,39.
Cell lines
Cell lines used in this study were purchased from the ATCC collection and were mycoplasma free.
RQ-PCR
RNA was reverse transcripted using MMLV (Invitrogen). We used a TaqMan assay to quantify TAL1 transcript with the following primers: TAL1 F: 5′-ACA-ATC-GAG-TGA-AGA-GGA-GAC-CTT-C-3′, TAL1 Probe: fam-5′-CTA-TGA-GAT-GGA-GAT-GGA-GAT-TAC-TGA-TG-3′-tamra, TAL1 R: 5′-ACG-CCG-CAC-AAC-TTT-GGT-G-3′, 40 cycles were run on ABI 7500HT (Applied Biosystem) as described40. TAL1 transcript quantification was performed after normalization with the housekeeping gene ABL using the ΔCt method and results calculated according to the following formula 2Δ(CtABL–CtTAL1).
TAL1 allelic expression analysis
Allelic expression was performed as previously described7. In brief, polymorphic markers in the 3′ UTR of the TAL1 gene were identified by PCR amplification and direct sequencing of 100 ng of genomic DNA. Allelic expression analysis was performed by PCR amplification and by direct sequencing of RT–PCR products from heterozygous patient samples. Three different PCRs were made to cover nine most frequent SNP among the 11 SNP previously described7.
Statistical analysis
ChIP. The power of t-test was estimated a priori using pwrR-package, and the expected variations between conditions evaluated. Differences in ChIP data between inserted and GL TAL1 alleles were analysed by unpaired t-test. Samples collections constituted of five or six technical replicates were first checked for normal distribution using Kolmogorov–Smirnov test and the equality of variances was tested using F test. Results of t-test are shown as two-tailed P values. The statistical power of executed t-tests was at least 80%. Errors bars on histograms represent s.e.m.
OS/DFS. Patients’ characteristics were compared using the Fisher’s exact test. Median comparisons were performed using the Mann–Whitney U-test. OS and DFS were calculated from the date of prephase initiation. Events accounting for DFS were induction failure and first relapse from any cause in first CR. OS and DFS were estimated by the Kaplan–Meier method and then compared by the log-rank test. All calculations were performed using the SPSS software, version 15.0 and the GraphPad Prism, La Jolla, CA, USA.
Ligation-mediated PCR
Genomic DNA was extracted using QIAamp deoxyribonucleic acid Blood mini kit according to the manufacturer’s instructions (Qiagen). DNA (500 ng) was used for LM-PCR; DNA was digested by DraI, EcoRV, PvuII, SmaI, SspI or StuI restriction endonucleases. Purified digested DNAs were ligated with an adapter (composed of two complementary primers GWA+ and GWAB−). A primary PCR amplification was performed using an adapter-specific primer (AP1) and primer specific for the different TCRβ gene segments (ext). A secondary PCR was performed using nested AP2 and TCRβ primers (int), and analysed on 1% agarose gel. Non-germline PCR products were purified and sequenced. The functional and non-functional V(D)J rearrangements from patient OC were obtained using Jβ2.7-extB/intB and AP1/2 primers. The breakpoints corresponding to the episomal insertion were then obtained using Vβ7.4-extB/intB, Dβ1-extA/intA and AP1/2 primers, and validated by direct PCR using TAL1.OC.1B and Vβ7.4-intB primers.
RT–PCR exon walking and 5′ RACE
Total RNA was extracted using a column-based system RNAeasy mini kit (Qiagen) according to the manufacturer’s instructions. Reverse transcription was performed with SuperScript III Reverse transcriptase (Invitrogen) and random primers (applied Biosytem). cDNAs were analysed by real-time quantitative PCR (RT–PCR) using power SybrGreen on an ABIPRISM 7500 (Applied Biosystems). All PCRs were performed in duplicate. 5′-RACE was performed using 2 μg of total RNA and the 5′/3′ RACE kit, 2nd generation (Roche). Modifications from the instruction manufacturer were the generation a poly(G) tailing of first strand cDNA and the use of an oligo d(C) anchor primer. PCR was performed using the Pfu Ultra II fusion HS DNA polymerase (Agilent technologies).
Genome editing in T-ALL cell line by type II CRISPR system
PEER T-ALL cells line were cultured in RPMI medium (Life Technologies) containing 20% fetal calf serum, 1% L-glutamine, 1% sodium pyruvate and 100 U ml−1 penicillin/streptomycin (Life Technologies) at 37 °C in the presence of 5% CO2. The day of transfection, 1 million cells were nucleofected according to the manufacturer’s instruction (Lonza), with 500 ng DNA donor sequence containing Neomycin-resistant gene and 2 μg of the Cas9/gRNA expression vector (Addgene #42230). The chimeric guide RNA targeted TAL1 insertion site, and was cloned according to Cong et al.41. One day after nucleofection, cells were plated in 96-wells plate at 104 cells per well and incubated in presence of 1,200 μg ml−1 geneticin G418 (Life Technologies) for 2 weeks. After selection and growing, a PCR was conducted to amplify the targeted region with genomic DNA derived from the surviving clones, and amplicons were separated on a 1% agarose gel then extracted with GEL/PCR clean up wizard (Promega) and sequenced (MWG-Biotech). CRISPR guide RNA: 5′-GAAAGACGTAACCCTACTTCC-3′.
Primers list is available on request.
Additional information
How to cite this article: Navarro, J.-M. et al. Site- and allele-specific polycomb dysregulation in T-cell leukaemia. Nat. Commun. 6:6094 doi: 10.1038/ncomms7094 (2015).
References
Teitell, M. A. & Pandolfi, P. P. Molecular genetics of acute lymphoblastic leukemia. Annu. Rev. Pathol. 4, 175–198 (2009).
Van Vlierberghe, P., Pieters, R., Beverloo, H. B. & Meijerink, J. P. Molecular-genetic insights in paediatric T-cell acute lymphoblastic leukaemia. Br. J. Haematol. 143, 153–168 (2008).
Sanda, T. et al. Core transcriptional regulatory circuit controlled by the TAL1 complex in human T cell acute lymphoblastic leukemia. Cancer Cell 22, 209–221 (2012).
Mouthon, M. A. et al. Expression of tal-1 and GATA-binding proteins during human hematopoiesis. Blood 81, 647–655 (1993).
Herblot, S., Steff, A. M., Hugo, P., Aplan, P. D. & Hoang, T. SCL and LMO1 alter thymocyte differentiation: inhibition of E2A-HEB function and pre-T alpha chain expression. Nat. Immunol. 1, 138–144 (2000).
Zhang, J. A., Mortazavi, A., Williams, B. A., Wold, B. J. & Rothenberg, E. V. Dynamic transformations of genome-wide epigenetic marking and transcriptional control establish T cell identity. Cell 149, 467–482 (2012).
Ferrando, A. A. et al. Biallelic transcriptional activation of oncogenic transcription factors in T-cell acute lymphoblastic leukemia. Blood 103, 1909–1911 (2004).
Hiom, K., Melek, M. & Gellert, M. DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocations. Cell 94, 463–470 (1998).
Agrawal, A., Eastman, Q. M. & Schatz, D. G. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature 394, 744–751 (1998).
Koch, F. et al. Transcription initiation platforms and GTF recruitment at tissue-specific enhancers and promoters. Nat. Struct. Mol. Biol. 18, 956–963 (2011).
Cuddapah, S. et al. Global analysis of the insulator binding protein CTCF in chromatin barrier regions reveals demarcation of active and repressive domains. Genome Res. 19, 24–32 (2009).
Cui, K. et al. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell 4, 80–93 (2009).
Zhou, Y. et al. Chromatin looping defines expression of TAL1, its flanking genes and regulation in T-ALL. Blood 122, 4199–4209 (2013).
McCabe, M. T. et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492, 108–112 (2012).
Cardoso, B. A. et al. TAL1/SCL is downregulated upon histone deacetylase inhibition in T-cell acute lymphoblastic leukemia cells. Leukaemia 25, 1578–1586 (2011).
Howe, S. J. et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 118, 3143–3150 (2008).
Brandt, V. L. & Roth, D. B. V(D)J recombination: how to tame a transposase. Immunol. Rev. 200, 249–260 (2004).
Clappier, E. et al. The C-MYB locus is involved in chromosomal translocation and genomic duplications in human T-cell acute leukemia (T-ALL), the translocation defining a new T-ALL subtype in very young children. Blood 110, 1251–1261 (2007).
Ntziachristos, P. et al. Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat Med. 18, 298–301 (2012).
Simon, C. et al. A key role for EZH2 and associated genes in mouse and human adult T-cell acute leukemia. Genes Dev. (2012).
Zhang, J. et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature 481, 157–163 (2012).
Grossmann, V. et al. EZH2 mutations and their association with PICALM-MLLT10 positive acute leukaemia. Br. J. Haematol. 157, 387–390 (2012).
Van Vlierberghe, P. & Ferrando, A. The molecular basis of T cell acute lymphoblastic leukemia. J. Clin. Invest. 122, 3398–3406 (2012).
Marculescu, R., Le, T., Simon, P., Jaeger, U. & Nadel, B. V(D)J-mediated translocations in lymphoid neoplasms: a functional assessment of genomic instability by cryptic sites. J. Exp. Med. 195, 85–98 (2002).
Mendes, R. D. et al. PTEN microdeletions in T-cell acute lymphoblastic leukemia are caused by illegitimate RAG-mediated recombination events. Blood 124, 567–578 (2014).
Marculescu, R. et al. Distinct t(7;9)(q34;q32) breakpoints in healthy individuals and individuals with T-ALL. Nat. Genet. 33, 342–344 (2003).
Vanura, K. et al. In vivo reinsertion of excised episomes by the V(D)J recombinase: a potential threat to genomic stability. PLoS Biol. 5, e43 (2007).
Reddy, Y. V. R., Perkins, E. J. & Ramsden, D. A. Genomic instability due to V(D)J recombination-associated transposition. Genes Dev. 20, 1575–1582 (2006).
Curry, J. D. et al. Chromosomal reinsertion of broken RSS ends during T cell development. J. Exp. Med. 204, 2293–2303 (2007).
Messier, T. L., O'Neill, J. P., Hou, S. M., Nicklas, J. A. & Finette, B. A. In vivo transposition mediated by V(D)J recombinase in human T lymphocytes. EMBO J. 22, 1381–1388 (2003).
Hacein-Bey-Abina, S. et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. New Engl. J. Med. 346, 1185–1193 (2002).
Hacein-Bey-Abina, S. et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302, 415–419 (2003).
Ntziachristos, P. et al. Contrasting roles of histone 3 lysine 27 demethylases in acute lymphoblastic leukaemia. Nature 514, 513–517 (2014).
Bracken, A. P. & Helin, K. Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nat. Rev. Cancer 9, 773–784 (2009).
Van der Meulen, J., Van Roy, N., Van Vlierberghe, P. & Speleman, F. The epigenetic landscape of T-cell acute lymphoblastic leukemia. Int. J. Biochem. Cell Biol. (2014).
Fenouil, R. et al. CpG islands and GC content dictate nucleosome depletion in a transcription-independent manner at mammalian promoters. Genome Res. 22, 2399–2408 (2012).
Roulland, S. et al. Follicular lymphoma-like B cells in healthy individuals: a novel intermediate step in early lymphomagenesis. J. Exp. Med. 203, 2425–2431 (2006).
Asnafi, V. et al. Analysis of TCR, pT alpha, and RAG-1 in T-acute lymphoblastic leukemias improves understanding of early human T-lymphoid lineage commitment. Blood 101, 2693–2703 (2003).
Asnafi, V. et al. Age-related phenotypic and oncogenic differences in T-acute lymphoblastic leukemias may reflect thymic atrophy. Blood (2004).
Bergeron, J. et al. Prognostic and oncogenic relevance of TLX1/HOX11 expression level in T-ALLs. Blood 110, 2324–2330 (2007).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Mochizuki-Kashio, M. et al. Dependency on the polycomb gene Ezh2 distinguishes fetal from adult hematopoietic stem cells. Blood 118, 6553–6561 (2011).
Delabesse, E. et al. TAL1 expression does not occur in the majority of T-ALL blasts. Br. J. Haematol. 102, 449–457 (1998).
Neiditch, M. B., Lee, G. S., Huye, L. E., Brandt, V. L. & Roth, D. B. The V(D)J recombinase efficiently cleaves and transposes signal joints. Mol. Cell 9, 871–878 (2002).
Acknowledgements
This work was supported by grants to BN laboratory from la Fondation pour la Recherche Médicale (#INE2003114116), Le Conseil Général des Bouches du Rhône, le Canceropôle PACA, CALYM consortium, l’Institut National du Cancer (INCa PLBIO09), la Fondation de France (#2008001490) and institutional grants from INSERM, CNRS and AMU. M.L. is a recipient of a fellowship from INCa (#ASC12035ASA). B.N. is the recipient of a CHRT INSERM/Assistance Publique-Hôpitaux de Marseille (HP-HM). Work in the Necker laboratory was supported by grants from the ‘Association Laurette Fugain’, the Institut National du Cancer and the ‘Comité départemental de la Ligue Contre le Cancer’. S.L.N. was supported by the ‘Institut National du Cancer (INCa-DHOS) and a grant from the ‘Association pour la Recherche sur le Cancer (ARC)’. R.F. is supported by a Centre National de la Recherche Scientifique grant. M.A.M. was supported by an ANR chromaTin and by the Fondation pour la Recherche Médicale (FRM AJE20130728183). The sequencing costs for ChIP-seq experiments were partially granted by an ESGI consortium grant from the Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 262055. E.D. gratefully acknowledges support from the Association pour la Recherche sur le Cancer and the Fondation pour la Recherche Médicale. We thank participants in the GRAALL-2003 and -2005 study groups for collecting and providing biological samples and data sets. The GRAALL was supported by grants P0200701 and P030425/AOM03081 from the Programme Hospitalier de Recherche Clinique, Ministère de l’Emploi et de la Solidarité, France and the Swiss Federal Government in Switzerland. Samples were collected and processed by the AP-HP ‘Direction de Recherche Clinique’ Tumour Bank at Necker-Enfants Malades. We are grateful to V. Lheritier for her help in collecting clinical samples, L Spinelli for statistical advice, T. Guignard for Macs2 analyses and C Pignon for technical help. We are grateful to Dr T Cathomen for expert advices in DNA editing.
Author information
Authors and Affiliations
Contributions
ChIP-seq experiments were performed and analysed by L.C.P., R.F., M.A.M., M.G., I.G.G. and J.-C.A.; allelic-ChIP experiments were performed and analysed by J.-M.N., L.C.P., M.L., M.K., C.P., D.P.-B. and E.D.; T-ALL sample characterizations were performed and analysed by A.T., J.-M.N., L.C.P., S.L.N., S.J., L.S., E.A.M. and V.A.; TALEN/CRISPR assays were performed and analysed by J.-M.N., M.L. and D.P.-B. with the advice of C.G. and B.M.; episomal reintegration characterization was performed by J.-M.N., E.A.M., S.H.-B.-A., S.J.H., H.B.G. and A.J.T.; survival analysis was carried out by A.T., H.D., E.A.M., N.I. and V.A.; V.A. and B.N. conceived and directed the project, and B.N. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-8, Supplementary Tables 1-2 and Supplementary References (PDF 1881 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Navarro, JM., Touzart, A., Pradel, L. et al. Site- and allele-specific polycomb dysregulation in T-cell leukaemia. Nat Commun 6, 6094 (2015). https://doi.org/10.1038/ncomms7094
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms7094
This article is cited by
-
Harnessing the MYB-dependent TAL1 5’super-enhancer for targeted therapy in T-ALL
Molecular Cancer (2023)
-
Current challenges in understanding the role of enhancers in disease
Nature Structural & Molecular Biology (2022)
-
A systematic review of recent trends in research on therapeutically significant l-asparaginase and acute lymphoblastic leukemia
Molecular Biology Reports (2022)
-
Oncogenic transcriptional program driven by TAL1 in T-cell acute lymphoblastic leukemia
International Journal of Hematology (2019)
-
Targeted genome editing in acute lymphoblastic leukemia: a review
BMC Biotechnology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.