Abstract
Las Cruces is a base-metal deposit in the Iberian Pyrite Belt, one of the world’s best-known ore provinces. Here we report the occurrence of major Pb-Ag-Au mineralization resulting from recent sub-surface replacement of supergene oxyhydroxides by carbonate and sulphide minerals. This is probably the largest documented occurrence of recent microbial activity producing an ore assemblage previously unknown in supergene mineralizing environments. The presence of microbial features in the sulphides suggests that these may be the first-described natural bacteriomorphs of galena. The low δ13C values of the carbonate minerals indicate formation by deep anaerobic microbial processes. Sulphur isotope values of sulphides are interpreted here as reflecting microbial reduction in a system impoverished in sulphate. We suggest that biogenic activity has produced around 3.1 × 109 moles of reduced sulphur and 1010 moles of CO2, promoting the formation of ca. 1.19 Mt of carbonates, 114,000 t of galena, 638 t of silver sulphides and 6.5 t of gold.
Similar content being viewed by others
Introduction
One of the principal ecological niches for extremophilic microbes is the sub-surface environment, where there is more opportunity for the existence of anaerobic life1. Here, the absence of oxygen is a limiting factor for heterotrophic processes. Consequently, lithoautotrophic bacteria create microdomains with metastable mineral assemblages that are inhibited under low-temperature, oxygenated surface conditions. Recent studies suggest that nearly the same amount of biomass is present in subterranean and sub-seafloor settings as on Earth’s surface1,2,3,4.
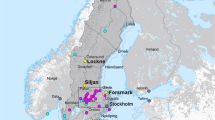
The contact between the Late Devonian–Early Carboniferous (ca. 360–330 Ma) volcanogenic massive sulphide deposit in the Las Cruces mine (Seville, southern Spain) and unconformably overlying Cenozoic sedimentary rocks of the Guadalquivir basin (Fig. 1a) hosts an unusual secondary mineral assemblage that includes galena, carbonates, iron sulphides, silver-rich sulphides and gold5,6,7,8. This assemblage forms a sub-horizontal rock layer, ~3–10 m thick, which caps the tilted primary massive sulphide lens. Beneath the layer is a large secondary cementation zone, up to 40 m thick, containing abundant chalcocite that constitutes the ore currently mined at Las Cruces, making it one of the richest copper deposits worldwide (17 Mt at 6.9% Cu)7. At the mine, the basement-cover contact is currently the locus of a major stratiform aquifer dominated by sulphate-bearing, calcium-bicarbonate, neutral to alkaline groundwaters.
(a) Regional geological map showing the location of the deposit. Adapted from ref. 10 with permission from Elsevier; (b) idealized sections of the typical gossan of the Iberian Pyrite Belt and of the secondary mineralized zone at Las Cruces (graphical scale, ca. 20 m). Outcropping ore deposits of the Iberian Pyrite Belt have well-formed gossans. Las Cruces is an exception, with a secondary zone covered by Cenozoic sediments. The quoted ages are depositional ages for the primary massive sulphides and the sediments but are formational ages of the gossan and secondary mineralization.
The massive sulphide orebody is similar to those elsewhere in the southern part of the Iberian Pyrite Belt9,10 that formed in the latest Famennian (ca. 360 Ma) and subsequently were folded and metamorphosed during the Variscan orogeny (330–300 Ma). The Las Cruces deposit differs, however, in occurring below 150 m of Late Tortonian–Messinian (ca. 7.2–5.3 Ma) sandstone and marl. This setting hindered its discovery and exploitation until 1994, but contributed greatly to the formation of the unusual mineral assemblages.
Other massive sulphide deposits of the Iberian Pyrite Belt that were exhumed in Miocene time (<23 Ma) underwent extensive sub-aerial supergene alteration and have classical secondary alteration zones11. The available data suggest that some of these secondary zones formed at 7–8 Ma (ref. 11). Here, sulphides were weathered and the leached caps were depleted of Cu and Zn but enriched in Fe, producing the well-known orange and red gossans that overlie most of the exposed volcanogenic massive sulphide deposits of the Pyrite Belt, and that contain abundant and visually distinctive haematite and goethite. Within these deposits, the contact between the cementation zone and the gossan is the locus of a yellowish layer, several metres thick, enriched in Pb, As, Sb, Hg, Ag and Au, and having significant amounts of barite and quartz. The lead is found in cerussite, anglesite, plumbojarosite or beudantite, whereas the silver seems to occur as small amounts of argentojarosite; gold is present as sub-microscopic inclusions within the goethite11. At Las Cruces, this typical gossan has been replaced by a younger sulphide-carbonate assemblage, which is, to our knowledge, foreign to sub-aerial supergene environments. We interpret this unusual rock as having formed due to sub-surface metabolism of anaerobic prokaryotes under the marl unit that seals the deposit.
Results
The secondary zone
In great contrast to the general behaviour of sub-aerially exposed orebodies in the Iberian Pyrite Belt and elsewhere, the gossan at Las Cruces shows a complex sequence of post-formational modifications (Fig. 1). Here, the original goethite- and haematite-rich assemblage is replaced by a siderite- and galena-rich rock (Red Rock), which in turn is replaced by a younger, highly heterogeneous rock (Black Rock) having variable proportions of coarse-grained (0.5–2 mm) calcite, fine-grained (<10 μm) galena and minute acicular to globular aggregates composed of greigite and smythite. The Black Rock also contains minor amounts of intergrown acanthite, sternbergite, proustite-xanthoconite, pearceite, jamesonite, cinnabar and cassiterite; gold-rich alloys are intergrown with the carbonates and galena. Barite and quartz are common gangue minerals in both the Black and Red rocks. Thin (<1 mm) sub-vertical veins of galena penetrate through the overlying marl12 and also cut the underlying massive sulphides. Blake8 first suggested that both the Black and Red rocks were of bacterial origin.
Bacteriomorphic structures
The Black Rock contains abundant, unbranched and twisted, thread-like aggregates of galena ca. 5–10 μm in length and ≤1 μm radius located on carbonate crystals (Fig. 2). These aggregates are morphologically similar to the microbial structures reported previously in the laboratory13 and in fossil14,15 geomicrobiological systems. The lack of external morphological features suggests that the galena precipitated as an extracellular polymer coating of bacteria as a result of metabolic sulphate reduction16. No previous records exist in natural systems of bacteriogenic structures composed of galena. Biogenic galena, to our knowledge, has only been described as a product of the bacterially mediated reduction of anglesite in discarded batteries17.
Stable isotope geochemistry
Carbon, oxygen and sulphur isotope analyses were obtained on the Red and Black rocks, as well as on the overlying marl and local groundwaters (Table 1). The δ13C and δ18O values of the siderite and calcite range from −42 to −18‰ versus V-PDB (δ13C) and +22.1 to +27.5‰ versus V-SMOW (δ18O). The δ34S values of the sulphides (versus V-CDT) are +11.9 to +25.9‰ for galena and +16.3 to +19.5‰ for greigite–smythite.
Discussion
The strict localization of the Red and Black rocks in the contact zone between the paleosurface and the deformed massive sulphides, and the presence of veins of galena cutting the overlying Tertiary sediments, indicate that these rocks are not related to the in situ submarine oxidation of the massive sulphides shortly after their formation at or near the seafloor, or by much later sub-aerial processes as proposed by Knight5. More probably, the Red and Black rocks formed after burial beneath the Late Tortonian–Messinian sediments8.
Both the presence of bacteria replaced by galena and the stable isotope data are consistent with replacement of the gossan being related to biogenic processes. The δ13Ccarbonate values are best interpreted as reflecting the mixing between 13C-depleted carbon and relatively isotopically heavy carbon (δ13C>−9‰), with this latter end member derived from dissolved inorganic carbon (DIC; defined as ΣC (CO2,aq+H2CO3+HCO3−)) in local groundwaters (Fig. 3a and Table 1). The most plausible source of the isotopically light carbon is the in situ biogenic oxidation of methane and other light hydrocarbons (Fig. 4); this interpretation is supported by the accumulations of light gas that are common in the Guadalquivir basin18. The ultimate origin of the light carbon may be thermal maturation of Palaeozoic shale within the underlying basement, a process that released gas that then ascended through fractures, accumulating below the sealing marl unit.
(a) Carbon–oxygen isotope composition of carbonates (sid, siderite; cc, calcite; ank, ankerite), overlying Tertiary sandstone and marl, and calculated δ18O-δ13C isotope composition of calcite in equilibrium with the local groundwaters (25 °C). Isotopic composition of the calcite in equilibrium with the water has been calculated from the tabulated δ18O-δ13C values together with the fractionation factors of Kim and O’Neil45 for oxygen and those of Romanek et al.46 for DIC. For comparison, data are shown for the Variscan (primary-ore related) hydrothermal siderite–ankerite assemblage occurring in VMS deposits of the Iberian Pyrite Belt. (b) Sulphur isotope composition compared with the δ34Ssulphate values of the local groundwater and δ34S values of the primary sulphides at Las Cruces19.
(a) General idealized crosssection of the deposit. The SE–NW distance is ~300 m. (b) Cartoon based on the section depicted in a showing the proposed genetic model for the deposit. Carbonate–sulphide rocks (Red and Black rocks) formed in the supergene alteration zone (gossan) capping an older massive sulphide deposit. The porous gossan accumulated methane or light hydrocarbons that acted as electron donors for microbial reduction of aqueous sulphate transported by groundwater. This process destabilized goethite/haematite and oxidized minerals (beudantite, cerussite), leading to the formation of siderite, calcite, galena and silver-bearing sulphides, together with coarse-grained gold that has almost completely replaced the former gossan. Scale bar, ~50 m.
Interpretation of the δ34S values for the sulphides is not straightforward, because the measured δ34S values are strikingly high compared with δ34Ssulphate values of the groundwaters currently flowing into the open pit of the Las Cruces mine (+6.3 to +15.2‰) and with those of the underlying massive sulphides (−6.8 to +8.2‰)8,19 (Fig. 3b and Table 1). However, these high δ34S values are similar to those of associated barite (+20.4‰)5. This situation is rather unusual for biogenically mediated, sulphide-rich ore systems20, because strongly negative δ34S values are generally regarded as prima facie evidence of biogenic sulphate reduction21,22, whereas small ΔSO4-H2S values are characteristic of thermochemical sulphate reduction23. At the low temperatures expected in the secondary environment of the Las Cruces deposit (≈<70–80 °C), sulphate reduction can only be mediated biogenically, typically by dissimilatory anaerobic processes, whereas abiotic sulphate reduction is kinetically inhibited23. Some bacteria are known to excrete reduced sulphur with small ΔSO4-H2S values24. However, we interpret the isotopically high δ34S values as recording fractionation in a system having excess organic matter (TOC>>SO42−) and high rates of reduction, whereby all of the sulphate was rapidly transformed into sulphide24,25. The isotope values reflect the complete consumption of sulphate during anaerobic oxidation of electron donors in a closed system26, a process that is consistent with the high δ34S values of the sulphides. The presence of iron monosulphides is indicative of a low-fS2 system and a limited availability of reduced sulphur. The ultimate origin of the groundwater sulphate is unknown, but it could have a seawater origin27, derived either from recent seawater intruding deep aquifers in the basin and upwelling along faults, or from the dissolution of accessory sulphate interbedded with the marl.
The mineral assemblages of siderite–calcite and sulphides are unstable under sub-aerial oxic conditions, and in the Las Cruces deposit could only have formed in an aqueous environment enriched in CO2 and, to some extent, in H2S. Thus, fluid–rock interactions should be able to produce three superimposed processes to form the observed assemblages: an increase of fCO2 with stabilization of carbonates; a redox change with reduction of Fe3+ to Fe2+, also related to the replacement of goethite/haematite by siderite; and sulphidization accompanied by the deposition of the sulphides.
The evolution of the gossan to the Red Rock must involve a complex set of reactions summarized as follows:



Microbes facilitating the classical reaction with methane and sulphate, producing CO2 and H2S (reaction 1), should be associated with another consortium of anaerobic bacteria that, in the presence of excess goethite, favour reaction (2) in which the goethite acts as an electron acceptor; this reaction is favoured due to its low free energy of formation28. Reactions (1) and (2) involve the oxidation of an electron donor, here represented by methane, but could well be organic molecules having low molecular weight. The low δ13C values exclude hydrogen as the main electron donor and point instead to the involvement of methane or light hydrocarbons.
The reduction of Fe3+ to Fe2+ associated with the replacement of goethite by siderite (reactions 2 and 3) is also probably due to bacterial activity. In surficial low-temperature environments, the reduction of Fe3+ to Fe2+ occurs predominantly by biogenic processes29,30. Abiogenic reduction of iron is unlikely to take place at Las Cruces because in surficial settings it has only been observed to occur in relationship with photoreduction31 and in some immature, organic matter-rich environments such as soils. In the latter setting, abiogenic reduction is associated with the oxidation of quinone-rich humic acids and phenolic compounds32, derived mainly from plant tissues. Even in the presence of organic compounds capable of reducing Fe3+, the extent of abiotic iron reduction is much less than that produced by biological reduction28 and, moreover, the actual role of phenolic compounds in direct abiotic reduction, or as electron shuttles from bacteria to Fe3+ mineral surfaces, is controversial.
The H2S generated during reaction (1) should increase fS2, leading to the destabilization of the cerussite and precipitation of galena

Finally, the formation of the Black Rock is related to replacement of the siderite by calcite:

In a strict sense, reactions (1) and (2) are not needed for the stabilization of the carbonate, because the flowing groundwater carries CO2 and, hence, reaction (1) could be replaced by

However, again the carbon isotope signatures indicate a significant input of carbon derived from the bacterial metabolization of methane/organic matter, therefore suggesting that reactions (1) and (2) are indeed relevant.
The precipitation of pyrite seems to be kinetically inhibited in the Black Rock. Instead, amorphous or poorly ordered greigite or smythite have precipitated. Although these minerals have been formed abiotically in the laboratory33,34, Kucha and Barnes35 and Raiswell and Plant36 proposed that the presence of such intermediate-valence monosulphides is directly related to biogenic precipitation. At Las Cruces, crystallization of these iron sulphides inhibited the formation of siderite and rendered calcite the stable carbonate in the presence of high aCa2+/aH+2. There is no available Ca2+ in the replaced gossan and, thus, we infer it is derived from the calcium-bearing groundwaters.
Formation of the complex Ag-As-Fe-Cu sulphosalts intergrown with the galena is interpreted as being related to destabilization of the Ag-bearing jarosite and to input of sulphur of biogenic derivation. We also interpret the formation of free gold as being linked to bacterial activity: during sulphidization of the gossan, sub-microscopic ‘invisible’ gold11 was transformed into coarse grains; by analogy, multiple lines of evidences have been presented for the biogenic formation of gold nuggets37,38.
Dissimilatory sulphate-reducing microbes have played a key role in the formation of many low-temperature stratiform ore deposits, with the reduced sulphur being derived from extensive biogenic reduction of dissolved sulphate22,39,40. Other sub-surface microbial activity is related to the secondary alteration of sulphides into oxides and carbonates41. During this supergene alteration, lithoautotrophic bacteria accelerate the dissolution of exposed ore deposits and promote the precipitation of secondary sulphides within the cementation zone, accelerating the process by up to five orders of magnitude above abiotic processes42,43. Biogenically mediated supergene precipitation of other sulphides has been described elsewhere, such as in the present-day formation of sphalerite biofilms in the Mike gold mine, in Nevada, USA23.
We propose that the unusual mineral assemblage found in the secondary zone of the Las Cruces ore deposit formed in a large natural bioreactor, where coupled microbial sulphate reduction and methane oxidation took place below a thick impermeable marl unit. The preferred site for such biogenic activity is where sulphate-bearing groundwaters interacted with a previously formed porous gossan, which was also the zone of gas accumulation beneath the sealing marl (Fig. 4). Under these conditions, anaerobic sulphate-reducing microbes were able to reduce the aqueous sulphate that then reacted in situ with the available metals; in fact, the metal assemblage found within this biotic zone is the same as that found in the gossans of the Iberian Pyrite Belt11. This process would be synchronous with the release of CO2 as a byproduct of the heterotrophic metabolism of anaerobic microbes; mixing of this carbon with DIC transported by the groundwater produced supersaturation in, and precipitation of, carbonates. The inferred high CH4/SO4 ratios should promote quick and nearly complete sulphate reduction and the anomalously high sulphur isotope signatures.
An estimation of the tonnages and metal grades of the Red and Black rocks, based on mineral compositions and abundances, suggests that the sub-surface microbial system at Las Cruces has modified the mineralogy of at least 2–6 km3 of rock, fixing at least 1.09 × 1010 moles of CO2 and 3.1 × 109 moles of H2S, and resulting in the formation of ca. 1.19 Mt of siderite and calcite, 114,000 t of galena, 638 t of silver sulphides and 6.5 t of free gold (Table 2). Such quantities reflect the existence of a vast underground microbial system, with the limiting factor in this system probably being the availability of electron acceptors.
The exact timing of the ore-forming process is difficult to constrain, because the assemblage lacks minerals suitable for accurate absolute dating. Geological relations suggest that the main gossan formation took place at 8–7 Ma (ref. 11) and that this gossan was covered by sediments at ca. 7.2–5.3 Ma; crosscutting relationships indicate that the deposition of galena postdated the formation of the underlying cementation zone and the overlying marl. Hence, the ore-forming event took place between burial at 7.2–5.3 Ma and the present. However, the microbially mediated mineralizing system has not changed significantly since burial and, thus, it may still be an active process.
Our model for the formation of secondary Pb-Ag-Fe sulphides and gold is different from those invoked for all known biogenically mediated ore-bearing systems. Equivalent secondary mineral assemblages are virtually unknown in the Earth’s crust; the only broadly comparable assemblages that have been reported are in the small Zapadno–Ozernoe mine, in the Russian Urals44. The formation of the large bioreactor in the subsurface at the Las Cruces deposit probably involves a wide community of competing chemolithotrophic microbes resulting in mineral-forming processes that are influenced and controlled by fluctuations in water input, temperature and availability of nutrients. Variations in any of these limiting factors and the nature of the aerobic/anaerobic interface probably control the size and resulting mineralogy of this vast underground ecosystem.
Methods
Sampling and preparation
Selected samples of ore, overlying sediments and waters were collected in situ from outcrops in the open pit; 1–3 g of individual minerals (carbonates and galena) were selected under the binocular microscope or separated magnetically (iron monosulphides); in the cases of finely intergrown assemblages, the gases were separated chemically following the procedures described below.
Isotope measurements
Carbon dioxide was obtained by reaction of the carbonates47,48 and DIC49 with 100% phosphoric acid. Water was analysed using the CO2–H2O equilibration method of Epstein and Mayeda50. For analysis of δD, an aliquot of water (0.5 μl) was injected into a ceramic column containing a glassy carbon tube at 1,400 °C to produce H2 and CO gases51. Sulphur isotopes of dissolved sulphates were measured after precipitation of BaSO4 from solution. SO2 was obtained from the barium sulphate as well as from the sulphides by combustion with V2O5 and O2 at 1,030 °C (ref. 52). Isotope measurements were carried out at the Stable Isotope Laboratory of the Instituto Andaluz de Ciencias de la Tierra (CSIC-UGR, Granada) with a Delta Plus XL (ThermoQuest, Bremen, Germany) mass spectrometer (elemental analysis–isotope ratio mass spectrometry) for sulphur, oxygen in sulphate, and water and deuterium isotope analyses, and a Delta Plus XP spectrometer for C and O isotopes in carbonates. Precision was calculated, after correction of the mass spectrometer daily drift, from data obtained on standards systematically interspersed in analytical batches, at better than ±0.1‰ for δ13C for carbonates and DIC, and for δ18O carbonates and water oxygen. Precision was better than ±0.2‰ for sulphate sulphur (δ34S) and for sulphate oxygen (δ18O), and better than ±2‰ for water hydrogen (δδD). The standard for reporting carbon measurements is VPDB (Vienna-PDB), for oxygen and hydrogen is VSMOW (Vienna Standard Mean Oceanic Water) and for sulphur is VCDT (Vienna Canyon Diablo Troilite).
Scanning electron microscope/energy-dispersive spectrometer analyses
Small, ca 2.5-mm grains of the Black Rock were analysed under a Jeol 5600-LV scanning electron microscope equipped with an Oxford Industries INCA X-sight energy-dispersive spectrometer at the Centro de Astrobiología. Backscattered and secondary electron images and energy-dispersive spectra were obtained on samples mounted on Al stubs and without coating (V=20 kV; I=85 μA, electron beam diameter=1 μm). Quantitative analysis performed on several (21) bacteriomorphic structures show that they are made of galena (mean 84.89±1.49% wt Pb, 15.11±1.02% wt S) with only negligible traces of other elements present.
Additional information
How to cite this article: Tornos, F. et al. Formation of recent Pb-Ag-Au mineralization by potential sub-surface microbial activity. Nat. Commun. 5:4600 doi: 10.1038/ncomms5600 (2014).
References
Shock, E. L. Minerals as energy sources for microorganisms. Econ. Geol. 104, 1235–1248 (2009).
Whitman, W. B., Coleman, D. C. & Wiebe, W. J. Prokaryotes: the unseen majority. Proc. Natl Acad. Sci. 95, 6578–6583 (1998).
Colwell, F. S. & D'Hondt, S. Nature and extent of the deep biosphere. Rev. Mineral Geochem. 75, 547–574 (2013).
Edwards, K. J., Becker, K. & Colwell, F. The deep, dark energy biosphere: intraterrestrial life on Earth. Ann. Rev. Earth Planet. Sci. 40, 551–568 (2012).
Knight, F. C. The Mineralogy, Geochemistry and Genesis of the Secondary Sulphide Mineralisation of the Las Cruces, Spain PhD Thesis, Univ. Cardiff434 p (2000).
Tornos, F., Velasco, F., Miguelez, N. G. & Escobar, J. M. inMineral Deposit Research for a High-Tech World –Proceed. 12th Biennial SGA Meeting eds Jonsson E.et al. 587–589Society for Geology Applied to Mineral Deposits (2013).
Doyle, M., Morrissey, C. & Sharp, G. inThe Geology and Genesis of Europe’s Major Base Metal Deposits eds Kelly C. G.et al. 381–390Irish Association for Economic Geology (2003).
Blake, C. The Mineralogical Characterisation and Interpretation of a Precious Metal-Bearing Fossil Gossan, Las Cruces, Spain PhD Thesis, Univ. Cardiff207 p (2008).
Leistel, J. M. et al. The volcanic-hosted massive sulphide deposits of the Iberian Pyrite Belt: review and preface to the special issue. Min. Deposit. 33, 2–30 (1998).
Tornos, F. Environment of formation and styles of volcanogenic massive sulfides: the Iberian Pyrite Belt. Ore Geol. Rev. 28, 259–307 (2006).
Velasco, F. et al. Supergene features and evolution of the gossans capping the massive sulphide deposits in the Iberian Pyrite Belt. Ore Geol. Rev. 53, 181–203 (2013).
Yesares, L., Sáez, R., Nieto, J. M., de Almodóvar, G. R. & Cooper, S. Supergene enrichment of precious metals by natural amalgamation in the Las Cruces weathering profile (Iberian Pyrite Belt, SW Spain). Ore Geol. Rev. 58, 14–26 (2014).
Sánchez-Andrea, I., Triana, D. & Sanz, J. L. Bioremediation of acid mine drainage coupled with domestic wastewater treatment. Water Sci. Technol. 66, 2425–2431 (2012).
Grenne, T. & Slack, J. F. Bedded jaspers of the Ordovician Løkken ophiolite, Norway: seafloor deposition and diagenetic maturation of hydrothermal plume-derived silica-iron gels. Min. Deposit. 38, 625–639 (2003).
Rasmussen, B. Filamentous microfossils in a 3,235-million-year-old volcanogenic massive sulphide deposit. Nature 405, 676–679 (2000).
Utgikar, V. P. et al. Inhibition of sulfate-reducing bacteria by metal sulfide formation in bioremediation of acid mine drainage. Environ. Toxicol. 17, 40–48 (2002).
Weijma, J., de Hoop, K., Bosma, W. & Dijkman, H. Biological conversion of anglesite (PbSO4) and lead waste from spent car batteries to galena (PbS). Biotechnol. Progr. 18, 770–775 (2002).
Melendez-Hevia, E. & Alvarez del Buergo, E. inTertiary Basins of Spain: The Stratigraphic Record of Crustal Kinematics eds Friend P. F., Dabrio C. J. 20–23Cambridge University Press (1996).
Velasco, F. et al. A new sulphur isotopic study of some Iberian Pyrite Belt deposits: evidence of a textural control on some sulphur isotope compositions. Min. Deposit. 34, 1–18 (1998).
Goodfellow, W. D. & Jonasson, I. R. Ocean stagnation and ventilation defined by δ34S secular trends in pyrite and barite, Selwyn Basin, Yukon. Geology 12, 583–586 (1984).
Fallick, A. E., Ashton, J. H., Boyce, A. J., Ellam, R. M. & Russell, M. J. Bacteria were responsible for the magnitude of the world class hydrothermal base metal sulfide orebody at Navan, Ireland. Econ. Geol. 96, 885–890 (2001).
Bawden, T. M. et al. Extreme S-34 depletions in ZnS at the Mike gold deposit, Carlin trend, Nevada: evidence for bacteriogenic supergene sphalerite. Geology 31, 913–916 (2003).
Machel, H. G. Bacterial and thermochemical sulfate reduction in diagenetic settings—old and new insights. Sed. Geol. 140, 143–175 (2001).
Rudnicki, M. D., Elderfield, H. & Spiro, B. Fractionation of sulfur isotopes during bacterial sulfate reduction in deep ocean sediments at elevated temperatures. Geochim. Cosmochim. Acta 65, 777–789 (2000).
Goldhaber, M. B. & Kaplan, I. R. Mechanisms of sulfur incorporation and isotope fractionation during early diagenesis in sediments of the Gulf of California. Marine Chem. 9, 95–143 (1980).
Johnson, C. A., Emsbo, P., Poole, F. G. & Rye, R. O. Sulfur- and oxygen-isotopes in sediment-hosted stratiform barite deposits. Geochim. Cosmochim. Acta 73, 133–147 (2009).
Fauré, G. Principles of Isotope Geology 2 edn Wiley & Sons (1986).
Zehnder, A. J. B. & Brock, T. D. Anaerobic methane oxidation: occurrence and ecology. Appl. Environ. Microbiol. 39, 194–204 (1980).
Neal, A. L. et al. Iron sulfides and sulfur species produced at hematite surfaces in the presence of sulfate-reducing bacteria. Geochim. Cosmochim. Acta 65, 223–235 (2001).
Lovley, D. R. Microbial Fe(III) reduction in subsurface environments. FEMS Microbiol. Rev. 20, 305–313 (1997).
Diez Ercilla, M., López Pamo, E. & Sánchez España, J. Photoreduction of Fe(III) in the acidic mine pit lake of San Telmo (Iberian Pyrite Belt): field and experimental work. Aquatic Geochem. 15, 391–419 (2009).
Pracht, J., Boenigk, J., Isenbeck-Schröter, M., Keppler, F. & Schöler, H. F. Abiotic Fe(III) induced mineralization of phenolic substances. Chemosphere 44, 613–619 (2001).
Schoonen, M. A. A. & Barnes, H. L. Reactions forming pyrite and marcasite from solution: II. Via FeS precursors below 100°C. Geochim. Cosmochim. Acta 55, 1505–1514 (1991).
Furukawa, Y. & Barnes, H. L. Reactions forming smythite, Fe9S11 . Geochim. Cosmochim. Acta 60, 3581–3591 (1996).
Kucha, H. & Barnes, H. L. Compounds with mixed and intermediate sulfur valences in pyrite from the Amelia mine, SW Wisconsin. Min. Deposit. 30, 78–81 (1995).
Raiswell, R. & Plant, J. The incorporation of trace elements into pyrite during diagenesis of black shales, Yorkshire, England. Econ. Geol. 75, 684–699 (1980).
Reith, F. et al. Nanoparticle factories: biofilms hold the key to gold dispersion and nugget formation. Geology 38, 843–846 (2010).
Southam, G., Lengke, M. F., Fairbrother, L. & Reith, F. The biogeochemistry of gold. Elements 5, 303–307 (2009).
Tornos, F., Solomon, M., Conde, C. & Spiro, B. F. Formation of the Tharsis massive sulfide deposit, Iberian Pyrite Belt: geological, lithogeochemical, and stable isotope evidence for deposition in a brine pool. Econ. Geol. 103, 185–214 (2008).
Kucha, H., Schroll, E. & Stumpfl, E. F. Fossil sulphate-reducing bacteria in the Bleiberg lead-zinc deposit, Austria. Min. Deposit. 40, 123–126 (2005).
Southam, G. & Saunders, J. A. The geomicrobiology of ore deposits. Econ. Geol. 100, 1067–1084 (2005).
Enders, M. S., Knickerbocker, C., Titley, S. R. & Southam, G. The role of bacteria in the supergene environment of the Morenci porphyry copper deposit, Greenlee County, Arizona. Econ. Geol. 101, 59–70 (2006).
Singer, P. C. & Stumm, W. Acidic mine drainage: the rate-determining step. Science 167, 1121–1123 (1970).
Belogub, E. V., Novoselov, C. A., Spiro, B. & Yakovleva, B. A. Mineralogical and S isotopic features of the supergene profile of the Zapadno-Ozernoe massive sulphide and Au-bearing gossan deposit, South Urals. Min. Magazine 67, 339–354 (2003).
Kim, S. T. & O’Neil, J. R. Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 61, 3461–3475 (1997).
Romanek, C. S., Grossman, E. L. & Morse, J. W. Carbon isotopic fractionation in synthetic aragonite and calcite—effects of temperature and precipitation rate. Geochim. Cosmochim. Acta 56, 419–430 (1992).
McCrea, J. M. On the isotopic chemistry of carbonates and a paleotemperature scale. J. Chem. Phys. 18, 849–857 (1950).
Al-Aasm, I. S., Taylor, B. E. & South, B. Stable isotope analysis of multiple carbonate samples using selective acid extraction. Chem. Geol. 80, 119–125 (1990).
Salata, G. G., Roelke, L. A. & Cifuentes, L. A. A rapid and precise method for measuring stable carbon isotope ratios of dissolved inorganic carbon. Marine Chem. 69, 153–161 (2000).
Epstein, S. & Mayeda, T. K. Variation of the 18O/16O ratio in natural waters. Geochim. Cosmochim. Acta 4, 213–224 (1953).
Sharp, Z. D., Atudorei, V. & Durakiewicz, T. A rapid method for determination of hydrogen and oxygen isotope ratios from water and hydrous minerals. Chem. Geol. 178, 197–210 (2011).
Révész, K., Haiping, Q. & Coplen, T. B. inMethods of the Reston Stable Isotope Laboratory: Reston, Virginia, U.S. Geological Survey Techniques and Methods (eds Révész K., Coplen T. B. Book 10, Sec. C, 33U.S. Geological Survey ( 2007).
Acknowledgements
This study was funded by project CGL2011-23207 of the SEIDI (Spain), the IPBSL project of the European Science Foundation and the ProMine project of the EU 7th Framework Program. We acknowledge the staff of Cobre Las Cruces, especially J.C. Baquero, I. Carrasco, C. Gomez, A. Francos and G. Obejero for granting access to the mine and for providing valuable data on geology and hydrogeology. We also thank R. Amils, C. Ayora, C. Conde, N.G. Miguelez, I. Sánchez-Andrea, J.C. Videira and N. White for fruitful discussions. Michael Russell offered thoughtful and constructive reviews of the original manuscript.
Author information
Authors and Affiliations
Contributions
F.T. and F.V. conceived of the study and together with J.M.E. did the field work, and collected and prepared the samples. F.V. and C.M.-S. performed the electron microprobe and scanning electron microscope analyses, respectively; A.D. did the isotope analyses. F.T., J.F.S. and F.V. took the lead in writing the paper. All authors contributed to the discussion.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tornos, F., Velasco, F., Menor-Salván, C. et al. Formation of recent Pb-Ag-Au mineralization by potential sub-surface microbial activity. Nat Commun 5, 4600 (2014). https://doi.org/10.1038/ncomms5600
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms5600
This article is cited by
-
The high-grade Las Cruces copper deposit, Spain: a product of secondary enrichment in an evolving basin
Mineralium Deposita (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.