Abstract
The existence of ~30 noncentrosymmetric metals (NCSM) suggests a contraindication between crystal structures without inversion symmetry and metallic behaviour. Those containing oxygen are especially scarce. Here we propose and demonstrate a design framework to remedy this property disparity and accelerate NCSM oxide discovery. The primary ingredient relies on the removal of inversion symmetry through displacements of atoms whose electronic degrees of freedom are decoupled from the states at the Fermi level. Density functional theory calculations validate this crystal-chemistry strategy, and we predict a new polar ruthenate exhibiting robust metallicity. We demonstrate that the electronic structure is unaffected by the inclusion of spin–orbit interactions, and that cation-ordered SrCaRu2O6 exhibits a large thermopower anisotropy (|ΔS⊥|~6.3 μV K−1 at 300 K) derived from its polar structure. Our findings provide chemical and structural selection guidelines to aid in the search of NCSM with enhanced thermopower anisotropy.
Similar content being viewed by others
Introduction
The metallic features in materials, which provide low-resistance channels for electrical conduction, lead to effective screening of local electric dipole moments1. Itinerant electrons disfavour both their formation and cooperative ordering2. In spite of the incompatibility between acentricity and metallicity, metallic materials that break the spatial parity operation mapping  were originally discussed in the 1960s by Matthias and then, later more rigorously by Anderson and Blount3. The first experimental identification4 of a candidate polar metal, the binary intermetallic V2Hf, was made a decade later. Since then, more noncentrosymmetric metals (NCSM) have been identified (Fig. 1) and found to exhibit unconventional optical responses5,6, magnetoelectricity7,8 and superconductivity9,10,11. Yet, they remain challenging to discover. The principal NCSM material classes are binary and ternary intermetallics and silicides. Intriguingly, few NCSM are oxides with the notable exceptions12,13,14,15 of a layered cuprate (Y1−xCaxSr2GaCu2O7±y), a geometrically frustrated pyrochlore (Cd2Re2O7), and recently LiOsO3 with a structural transition similar to ferroelectric LiNbO3. (Degenerately doped dielectrics like BaTiO3−δ (ref. 16) and ZnO:Al17, although exhibiting metallic resistivity, are not intrinsically metallic and NCS—their conductivity is high because of deviations from ideal stoichiometry.) Figure 1 suggests that NCSM tend to require cations with large atomic masses, but this is not always the case, for example, Mg2Al3. The absence of reliable crystal-chemistry guidelines and the limited understanding of the microscopic origin of inversion symmetry-lifting displacements in metals poses a serious challenge for their discovery: how does one explain, let alone design, cooperative acentric atomic displacements in crystalline oxide conductors?
were originally discussed in the 1960s by Matthias and then, later more rigorously by Anderson and Blount3. The first experimental identification4 of a candidate polar metal, the binary intermetallic V2Hf, was made a decade later. Since then, more noncentrosymmetric metals (NCSM) have been identified (Fig. 1) and found to exhibit unconventional optical responses5,6, magnetoelectricity7,8 and superconductivity9,10,11. Yet, they remain challenging to discover. The principal NCSM material classes are binary and ternary intermetallics and silicides. Intriguingly, few NCSM are oxides with the notable exceptions12,13,14,15 of a layered cuprate (Y1−xCaxSr2GaCu2O7±y), a geometrically frustrated pyrochlore (Cd2Re2O7), and recently LiOsO3 with a structural transition similar to ferroelectric LiNbO3. (Degenerately doped dielectrics like BaTiO3−δ (ref. 16) and ZnO:Al17, although exhibiting metallic resistivity, are not intrinsically metallic and NCS—their conductivity is high because of deviations from ideal stoichiometry.) Figure 1 suggests that NCSM tend to require cations with large atomic masses, but this is not always the case, for example, Mg2Al3. The absence of reliable crystal-chemistry guidelines and the limited understanding of the microscopic origin of inversion symmetry-lifting displacements in metals poses a serious challenge for their discovery: how does one explain, let alone design, cooperative acentric atomic displacements in crystalline oxide conductors?
Known compounds differentiated by crystal class following the NCS classification scheme introduced in Halasyamani and Poeppelmeier65 include 4d and 5d transition metal intermetallics, carbides or silicides. Oxide compounds are scarce.
In this work, we present a microscopic understanding for NCSM, leading to a design principle that we use in the theoretical prediction of a polar NCSM transition metal (M) oxide. We formulate a two-step material selection process based on a weak electron–lattice coupling principle that considers the symmetry and atomic species participating in the inversion-lifting lattice modes, and the susceptibility of the cations’ electronic configurations to a metallic state. We show that two k≠0 lattice modes describing rotations of MO6 octahedra induce polar A-cation displacements in the layered and cation-ordered (Sr,Ca)Ru2O6 perovskite, for which experimental solid solutions18 are metallic. Because the microscopic mechanism is independent of the details of the states at the Fermi level, unlike conventional dipolar interactions that are essential to stabilizing acentric displacements of second-order Jahn–Teller cations in dielectrics19, the design strategy is readily transferable to other transition metal chemistries. We show that spin–orbit interactions (SOI) do not alter the electronic ground state, and that the unusual coexistence of a polar axis and metallicity produces a novel correlated material possessing a highly anisotropic thermopower response (|ΔS⊥|~6.3 μV K−1 at 300 K) with nearly isotropic electrical conductivity.
Results
Design of a metallic oxide without inversion
Our design strategy originates from Anderson’s work3 on ‘ferroelectric metals,’ where he writes that “while free electrons screen out the electric field [in materials] completely, they do not interact very strongly with the transverse optical phonons and the Lorentz local fields [that] lead to ferroelectricity, since umklapp processes are forbidden as k→0.” We recast this observation into an operational principle that states: the existence of any NCSM relies on weak coupling between the electrons at the Fermi level, and the (soft) phonon(s) responsible for removing inversion symmetry. An essential, implicit, materials constraint is that the low-energy electronic structure derives from an electron count giving partial band occupation, which may be obtained by judicious selection of the cation chemistries.
We identify three displacive routes fulfilling the weak-coupling hypothesis in solid-state systems, specified by the symmetry behaviour of the lattice phonons that would remove inversion symmetry in a centrosymmetric metal and yield a NCS crystal structure (Table 1). It should be noted that an order–disorder mechanism could also be operative; the classification of such symmetry breaking is consistent with our scheme; the caveat being that the soft phonon is replaced by an order parameter that describes atomic site occupancy. In either case the same set of irreducible representations may be used.
The third approach, which we focus on here, resembles a condition that supports a novel ‘improper’ mechanism20,21 for ferroelectricity that has been exploited to design artificial polar oxides with sizeable electric polarizations by heterostructuring non-polar dielectrics. Note that throughout we choose to refer to these materials as NCS metals rather than ‘ferrroelectric metals’ as articulated by Anderson and recently others to eliminate misconceptions about the presence of switchable spontaneous electric polarizations in metals, and more accurately describe the crystallographic–electronic function of the materials. In this way, one can generally and completely classify all metals without inversion symmetry and not solely those of the polar subset of NCS structures (Fig. 1).
Within this framework, the design of a NCSM oxide using geometric-induced displacements requires an oxide class exhibiting coupled zone-boundary phonons that lift inversion symmetry. Here we choose orthorhombic AMO3 perovskites with corner-connected MO6 octahedra that are rotated about each Cartesian axis; the ‘tilt’ pattern is obtained and described by two phonons with k=(½, ½, 0) and (½, ½, ½) wavevectors relative to the cubic aristotype22. The a+b−c− MO6 rotation pattern in the presence of layered A-cation order along a [001] direction, for example, accessible through synthetic bulk chemistry routes23,24 or heteroepitaxial thin-film growth methods25, is sufficient to produce a polar structure26,27. The chemical species are selected given the constraints that the rotations of octahedra are necessary—determined by the A and M cation size mismatch in perovskites—and that the electronic configuration of the M cation results in a nonzero density-of-states at the Fermi level (EF). These requirements should be satisfied for ordered (Sr,Ca)Ru2O6, since both bulk SrRuO3 and CaRuO3 are experimentally found in the a+b−c− Glazer tilt pattern and also have partially occupied low-energy Ru 4d t2g orbitals.
Ground-state structure
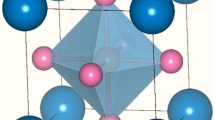
Figure 2 depicts the polar Pmc21 ground state crystal structure of (Sr,Ca)Ru2O6 obtained from density functional theory calculations. (See Supplementary Table 1 for the crystallographic data.) The extended RuO6 structure is highly rotated, adopting the targeted orthorhombic tilt pattern with rotations of adjacent octahedra out-of-phase about the [011] direction and in-phase about the layering [100] direction (Fig. 2a). The Ru–O–Ru bond angles are 157.1° and 145.6° (perpendicular to the CaO/SrO layers) and 150.4° (within the layers). The stabilization of this rotation pattern is understood on the grounds that the π* band, which is nearly full because of the  configuration, is shifted to lower energy by the electronegative Ru4+ cation28.
configuration, is shifted to lower energy by the electronegative Ru4+ cation28.
Crystal structure of cation-ordered (Sr,Ca)Ru2O6 with the a+b−b− RuO6 tilt pattern (a). The off-centre Ru4+ distortions (bond lengths in Å) produce local dipoles that cooperatively order along the c direction (b), removing the mirror plane perpendicular to the A-site cation order. The local coordination environment for (c) Sr (tetra-capped trigonal prism) and (d) Ca (bi-capped trigonal prism) reveals that large A-site distortions from the ideal 12-fold coordination occur in opposite directions (arrows), along the polar c axis.
(Sr,Ca)Ru2O6 exhibits small off-centre Ru distortions (Fig. 2b) and large A-site cation displacements (Fig. 2c,d). Acentric B-site displacements are common to TMs with d0-electronic configurations in octahedral coordinations; however, for dn, n≥1, such polar displacements are largely disfavoured, that is, the energetic gain due to dπ−pπ metal–oxygen bonding decreases on filling the t2g orbital29. Nonetheless, we find they occur for d4 Ru4+, displacing the metal centre nearly towards an edge (~0.03 Å), staying in the (110) plane (see Supplementary Table 2). This results in one short, two long and three medium Ru–O bonds (Fig. 2b), which are close to the average value found experimentally in the Sr0.5Ca0.5Ru2O3 solid solution30. The Ca and Sr atoms make large polar displacements (0.29 and 0.21 Å, respectively) along the [001] and [ ] directions (see Supplementary Table 3), resulting in distorted polyhedra (Fig. 2c). We explore these displacements and their effect on the electronic structure in more detail below.
] directions (see Supplementary Table 3), resulting in distorted polyhedra (Fig. 2c). We explore these displacements and their effect on the electronic structure in more detail below.
Electronic properties
Figure 3 shows the total and partial densities-of-states (DOS) for the polar NCSM (Sr,Ca)Ru2O6 without (Fig. 3a) and with (Fig. 3b) SOI. The valence band is composed largely of O 2p states hybridized with Ru 4d states, with the oxygen states predominately found in regions below the EF. Consistent with low-spin Ru4+, the large peaks in the DOS near EF are caused by the fairly flat Ru t2g bands, whereas the strongly hybridized eg orbitals form broader bands in the conduction band beginning near 1.5 eV. We find that without SOI, weakly dispersive t2g bands lead to a large number of states at the Fermi level, that is, N(EF)~2.2 eV per spin per Ru atom indicating robust metallic behaviour (Fig. 3a), despite a ~0.75 eV band gap present in both spin channels far above EF (0.5 eV). It is also ferromagnetic (0.914 μB f.u.−1) with ~0.35 μB per Ru atom; the remaining magnetization is distributed among the oxygen ligands due to the itinerant spin-polarized electrons. Using a simple modified Weiss formula31, we calculate a Curie temperature Tc=47 K (27 K applying Anderson’s rescaling32) in good agreement with experimental data18 on solid solution Sr0.5Ca0.5RuO3 (Tc=57 K).
Since SOI play a significant role in determining the orbital structure in 3d transition metal oxides33,34, we calculate the DOS with SOI (Fig. 3b). Although the SOI enable the spin-up and spin-down bands to mix, we find an electronic structure similar to that without the interactions. The Ru 4d-band occupation at EF is weakly modified and the gap in the conduction band is slightly reduced. Nonetheless, the weakly dispersed t2g states persist, and (Sr,Ca)Ru2O6 remains metallic [N(EF)~4.36 eV per Ru atom] consistent with SOI calculations on bulk SrRuO3 (ref. 35), where spin–orbit coupling also does not greatly alter the electronic structure.
Origin of acentricity
For purposes of evaluating the design approach and the proposed geometric-induced inversion symmetry-breaking displacements, we perform a group theoretical analysis36,37 of the Pmc21 structure with respect to a fictitious prototypical centrosymmetric phase (P4/mmm symmetry), reducing the polar structure into a set of symmetry-adapted modes associated with different irreducible representations of the P4/mmm phase (see Supplementary Fig. 1 and Supplementary Table 4). Note that the hypothetical structure would be related to an experimentally accessible high-temperature phase, assuming that the crystal would not first decompose. The normalized distortion vector is  , where
, where  corresponds to a polar ir-active mode, and
corresponds to a polar ir-active mode, and  and
and  describe k≠0 in-phase and out-of-phase rotation modes, respectively.
describe k≠0 in-phase and out-of-phase rotation modes, respectively.
The amplitude and energetic contribution of each irrep in the polar structure provide insight into the stability of the NSCM (Fig. 4). Although the zone-centre polar mode transforming as irrep  is weakly unstable in the high-symmetry P4/mmm structure (see Supplementary Fig. 2 and Supplementary Table 5), that is, characterized by a negative quadratic energy surface with respect to the increasing amplitude of the polar distortion (Fig. 4a), its energetic contribution to the ground state structure is two orders of magnitude smaller than that obtained from the octahedral rotation modes (Fig. 4b). To show that the geometric RuO6 rotations drive the polar Sr and Ca displacements (Fig. 4a, inset), circumventing the contraindication between metallicity and the ir-mode, we map the total energy evolution of (Sr,Ca)Ru2O6 with increasing amplitude of the ir-polar mode at different fixed values of the a+b−b− tilt pattern described by (
is weakly unstable in the high-symmetry P4/mmm structure (see Supplementary Fig. 2 and Supplementary Table 5), that is, characterized by a negative quadratic energy surface with respect to the increasing amplitude of the polar distortion (Fig. 4a), its energetic contribution to the ground state structure is two orders of magnitude smaller than that obtained from the octahedral rotation modes (Fig. 4b). To show that the geometric RuO6 rotations drive the polar Sr and Ca displacements (Fig. 4a, inset), circumventing the contraindication between metallicity and the ir-mode, we map the total energy evolution of (Sr,Ca)Ru2O6 with increasing amplitude of the ir-polar mode at different fixed values of the a+b−b− tilt pattern described by ( ) (Fig. 4c). We find that with increasing amplitude of the a+b−b− tilt pattern, the negative curvature about the origin vanishes; the energy landscape evolves to a parabola with a single energy minimum, indicating the hardening (stabilization) of the polar phonon mode at finite amplitude induced by the geometric RuO6 octahedral rotations, which fully accounts for the loss of inversion symmetry.
) (Fig. 4c). We find that with increasing amplitude of the a+b−b− tilt pattern, the negative curvature about the origin vanishes; the energy landscape evolves to a parabola with a single energy minimum, indicating the hardening (stabilization) of the polar phonon mode at finite amplitude induced by the geometric RuO6 octahedral rotations, which fully accounts for the loss of inversion symmetry.
(a) Polar (ir-active) mode with  symmetry. The inset depicts the polar cation displacement pattern; apical oxygen and (small) Ru displacements are omitted for clarity. (b) In-phase and out-of-phase RuO6 rotational modes. (c) Normalized energy gain obtained by increasing amplitude of the polar displacements at fixed percentages of the total a+b−b− tilt pattern found in the ground-state structure, that is, described by the combination
symmetry. The inset depicts the polar cation displacement pattern; apical oxygen and (small) Ru displacements are omitted for clarity. (b) In-phase and out-of-phase RuO6 rotational modes. (c) Normalized energy gain obtained by increasing amplitude of the polar displacements at fixed percentages of the total a+b−b− tilt pattern found in the ground-state structure, that is, described by the combination  . The inset depicts the 100% rotational distortions. (d) Change in the position of the Sr and Ca conduction band centre of mass (
. The inset depicts the 100% rotational distortions. (d) Change in the position of the Sr and Ca conduction band centre of mass ( ) and (e) variation in the number of states at the Fermi level [ΔN(EF)] normalized to the value without the polar distortion. The ferromagnetic state is stable for polar displacements larger than ~0.25 Å.
) and (e) variation in the number of states at the Fermi level [ΔN(EF)] normalized to the value without the polar distortion. The ferromagnetic state is stable for polar displacements larger than ~0.25 Å.
Figure 4d shows there are minor changes in the position of the band centre of mass for the principal atoms involved in the polar mode, that is, the Ca (4s3d) and Sr (5s4d) states, which are located ~6.2 eV above EF in the conduction band. The relative change in the number of states at the Fermi level (ΔN(EF), Fig. 4e) is also small with respect to the centrosymmetric structure and increasing amplitude of the polar mode, confirming the weak interaction between the inversion symmetry-lifting displacements and the low-energy electronic structure. The conduction bands marginally shift to higher energy (~0.06 eV) and the change in N(EF) is ~±5%, validating the proposed NCSM design guidelines. Interestingly, the polar mode activates the ferromagnetic ground state observed by the exchange split electronic structure that appears around ~0.25 Å in Fig. 4e, indicative of large spin–phonon coupling38,39.
Thermoelectric response
Owing to the orthorhombicity in (Sr,Ca)Ru2O6, the recent interest in thermoelectric properties of perovskite oxide superlattices40, and the fact that degenerately doped ferroelectrics with polar axes exhibit unusual thermoelectric properties41,42,43, we evaluate the thermopower anisotropy at 300 K using Botlzmann transport theory within the constant scattering time approximation.
Figure 5 shows the relevant thermoelectric properties in the chemical potential range varying from −0.25 to 0.25 eV. The average thermopower  , denoted by the broken line in Fig. 5a, ranges from 14 to −18 μV K−1 with two sign changes near μ≃−0.21 and μ≃0.07 eV, indicating a change in the dominant charge carriers from holes to electrons and then from electrons to holes, respectively. We also note that above μ≃0.16 eV, the thermopower components along the polar c direction dominate the thermal properties.
, denoted by the broken line in Fig. 5a, ranges from 14 to −18 μV K−1 with two sign changes near μ≃−0.21 and μ≃0.07 eV, indicating a change in the dominant charge carriers from holes to electrons and then from electrons to holes, respectively. We also note that above μ≃0.16 eV, the thermopower components along the polar c direction dominate the thermal properties.
(Sr,Ca)Ru2O6 transport properties (left panels) as a function of the chemical potential μ: (a) thermopower S, (b) conductivity σ and (c) the power factor PF. Red, blue and green lines are the transport properties along the a, b and the polar c axis, respectively. The dashed line indicates the total behaviour, given as an average of the three principle components. Anisotropy (right panels) along (d) the ab plane (ΔS||) with (blue) and without (magenta) polar displacements; the difference of the two curves is depicted in (e). (f) The anisotropy between the polar c axis and the ab plane (ΔS⊥), and (g) the difference between the two curves.
The conductivity σ is found to oscillate in a narrow window over the chemical potential range considered, and all the three components resolved along the crystallographic axes exhibit similar behaviour (Fig. 5b). Above μ≃0.05 eV, where the Seebeck coefficient along the polar axis is extremized, the conductivity along the c axis (σc) begins to exceed that along the other directions.
Figure 5c shows the total and crystal axis-decomposed power factor PF=σS2. The total power factor exhibits a pronounced local maximum, PF≃0.7 μW K−2 cm−1, near μ=−0.12 eV (hole doping h=3 × 1021 cm−3), and two other less pronounced local maxima, PF≃0.35 μW K−2 cm−1 at μ~0.05 eV and μ~0.18 eV, which are negligible when compared with thermoelectric materials found in applications (20–50 μW K−2 cm−1)44. It is interesting, however, that the maximum at μ~0.18 eV (electron doping of n=6 × 1021 cm−3) is in the region where the Seebeck coefficient and the conductivity along the polar axis both dominate the transport behaviour along the other directions.
To show that this anisotropy arises from the polar displacements and not the orthorhombic lattice symmetry of (Sr,Ca)Ru2O6, we calculate the thermopower anisotropy in the ab plane as  (Fig. 5d), and the thermopower anisotropy along the polar c axis as
(Fig. 5d), and the thermopower anisotropy along the polar c axis as  (Fig. 5f), for the equilibrium Pmc21 structure and for a hypothetical structure with the polar atomic displacements described by irrep
(Fig. 5f), for the equilibrium Pmc21 structure and for a hypothetical structure with the polar atomic displacements described by irrep  fully removed.
fully removed.
When we remove the polar displacements, we find that ΔS|| is modified for all chemical potentials examined, except at 0 eV. The difference between the two cases ΔS|| ranges between ±15 μV K−1 (Fig. 5e). The thermopower anisotropy along the polar c axis, ΔS⊥, behaves as ΔS||, except for a sign change at 0 eV: The difference in the c axis anisotropy, δS⊥, also ranges between ±15 μV K−1 for the two structures (Fig. 5g). Consistent with our design guidelines, at μ=0 eV, the change in the thermopower anisotropy is essentially zero, indicating weak coupling between the states at the Fermi level and the polar atomic displacements that lift inversion symmetry. The role of the polar distortions becomes even more evident by analysing the anisotropy change in the bc plane (see Supplementary Fig. 3) where the lattice parameters are identical. The removal of polar displacements is found to produce a shift relative to the chemical potential below μ=0.18 eV. Above this value, we find that the anisotropy in the bc plane is small in the structure without the polar distortions, whereas it is maximized in the equilibrium structure. Such behaviour is also observed in Fig. 5f over the same electron-doped region. The magnitude of the anisotropy, |δS⊥|, in the ground state structure exceeds that of the phase with the polar displacements removed.
Figure 6 shows the computed temperature-dependent thermopower S as a function of chemical potential μ=0 and 0.18 eV, which is selected because the power factor (σS2) exhibits a local maximum that is due mainly to the Seebeck coefficient along the polar axis, Sc (Fig. 5c). Due to identical lattice constants along the b and c axes in (Sr,Ca)Ru2O6, Sb and Sc have similar behaviour for μ=0 eV. The total thermopower,  , ranges from ~2.5 to −7 μV K−1 and exhibits parabolic behaviour with a maximum around 550 K. The thermopower anisotropy along the polar c axis, ΔS⊥, exhibits the same behaviour. At μ=0 eV, the thermopower anisotropy is relatively large (|ΔS⊥|~6.3 μV K−1), reflected in the 300 K representation of the second-rank Seebeck tensor (inset) appearing as a hyperboloid with two sheets.
, ranges from ~2.5 to −7 μV K−1 and exhibits parabolic behaviour with a maximum around 550 K. The thermopower anisotropy along the polar c axis, ΔS⊥, exhibits the same behaviour. At μ=0 eV, the thermopower anisotropy is relatively large (|ΔS⊥|~6.3 μV K−1), reflected in the 300 K representation of the second-rank Seebeck tensor (inset) appearing as a hyperboloid with two sheets.
Temperature-dependent thermopower along the a, b and c axes at (a) μ=0 eV and (b) μ=0.18 eV. The shaded area indicates the ferromagnetic phase below the calculated Curie temperature Tc~50 K. The average thermopower is calculated as  . The insets depict the 300 K quadric surface representations of the second-rank Seebeck tensor, revealing enhanced anisotropy on doping along the polar axis.
. The insets depict the 300 K quadric surface representations of the second-rank Seebeck tensor, revealing enhanced anisotropy on doping along the polar axis.
For μ=0.18 eV the scenario is different.  has a quasi-linear behaviour from low to high temperature: Sa, Sb and Sc all become positive. Over the entire temperature range considered, the thermopower along the polar axis Sc dominates. At 300 K (broken vertical line), the second-rank Seebeck tensor quadric surface becomes an elongated ellipsoid, flattened along the c direction. Accordingly the thermopower anisotropy at 300 K becomes ~5.8 μV K−1.
has a quasi-linear behaviour from low to high temperature: Sa, Sb and Sc all become positive. Over the entire temperature range considered, the thermopower along the polar axis Sc dominates. At 300 K (broken vertical line), the second-rank Seebeck tensor quadric surface becomes an elongated ellipsoid, flattened along the c direction. Accordingly the thermopower anisotropy at 300 K becomes ~5.8 μV K−1.
Discussion
The thermoelectric behaviour as a function of doping can be understood by examining the electronic band structures of the equilibrium Pmc21 structure with that of the hypothetical (Sr,Ca)Ru2O6 system with the polar atomic displacements removed (Fig. 7). Inspection of the electronic band structure along the lines of symmetry X–S (corresponding to b in real space) and S–R (corresponding to the polar c axis) reveals strongly hybridized Ru 4d–O 2p bands dispersing from −0.2 eV to 0.3 eV, where the electron contribution dominates until ~0.07 eV above the Fermi level (Fig. 7a), explaining the sign change of  near μ≃0.07 eV in Fig. 5a.
near μ≃0.07 eV in Fig. 5a.
In the left (right) panel the bands structure for the non-magnetic layered (Sr,Ca)Ru2O6 (without  displacements). The highlighted bands are mainly affected by the polar displacements. The red (lower energy) bands are partially occupied in the equilibrium structure. The shaded path corresponds to a k-space trajectory parallel to the polar axis. Brillouin zone labels: Γ (0, 0, 0), X (π/2, 0, 0), S (π/2, π/2, 0), R (π/2, π/2, π/2).
displacements). The highlighted bands are mainly affected by the polar displacements. The red (lower energy) bands are partially occupied in the equilibrium structure. The shaded path corresponds to a k-space trajectory parallel to the polar axis. Brillouin zone labels: Γ (0, 0, 0), X (π/2, 0, 0), S (π/2, π/2, 0), R (π/2, π/2, π/2).
In addition, the polar displacements largely modify the band structure along S−R in the energy range 0.05–0.30 eV. In the absence of the polar mode, the band near 0.16 eV (arrow in Fig. 7), which is derived from the oxygen 2p states in the CaO and SrO planes, shifts to lower energy and broadens. In the equilibrium structure, this band along S–R is higher in energy than the band which changes dispersion along Γ–X (energy range 0.05–0.18 eV); however, when the polar displacements are removed, the oxygen-derived band along S–R becomes lower than that of the band centre of mass along Γ–X. It is the dependence of these two bands on the amplitude of the polar atomic displacements that leads to the different behaviour appearing in Fig. 5f for μ=0.18 eV.
We find that the 300 K thermopower anisotropy of (Sr,Ca)Ru2O6 is close to that of centrosymmetric YBa2Cu3O7−δ (YBCO, ~7–10 μV K−1)45,46, which exhibits strong thermal and electrical anisotropy owing to the two-dimensional (2D) crystal structure. The thermopower anisotropy in other metallic systems, for example, the hexagonal delafossite oxides PdCoO2 and PtCoO2 (ref. 47), also derive from the 2D layered topology of the system, and so present large Seebeck coefficients along the trigonal axis, that is, the axis which is most insulating. In contrast, the thermopower anisotropy in (Sr,Ca)Ru2O6 is not related to the dimensionality of the system, but rather to the existence of a polar axis along which the electrical conductivity is highest.
The peculiar thermopower anisotropy in (Sr,Ca)Ru2O6 dictates that the electric field resulting from an applied heat flux to the material will be non-collinear. This property is a fundamental feature for any anisotropic thermoelectric devices48. It enables the heat flux to be measured in a geometry perpendicular to the induced electrical current, specifically at locations where the temperatures are equal (see Supplementary Fig. 4 and Supplementary Note 1). Given the metallic conductivity of (Sr,Ca)Ru2O6 and that the relaxation time is small in comparison with that of semiconductors or insulators already finding use in thermoelectric devices, this polar NCSM would enable sensing on sub-nanosecond timescales (comparable to YBCO46). New applications of these oxide materials could be found in ultrafast thermoelectric devices46,48, where stability under extreme conditions, speed, and the ability to measure heat fluxes of high density are key requirements, for example, thermal (heat) radiation detectors. Moreover, because of the compatibility of perovskite oxides with Si-based CMOS technologies49, we anticipate that these designed NCSMs will more readily find integration and device development than previously identified materials with large anisotropic thermoelectric responses.
In summary, we designed a polar noncentrosymmetric ruthenate conductor and described how to eliminate the incompatibility between metallicity and acentricity in complex transition metal oxides. We articulated a paradigm for which new NCMS may be found, and showed that (Sr,Ca)Ru2O6 exhibits Seebeck coefficients with anisotropy derived from the polar structure. We believe that other polar metals could exhibit such anisotropic thermal properties; the important discriminating feature, however, is the k-space evolution of the electronic structure with the real space polar distortion. Broadly, such thermopower anisotropy may also be found in any NCSM, for example, in compounds which are chiral but non-polar, but continued studies are required to clarify the extent of the response. We hope this work motivates the synthesis of new materials and the discussion of new applications where highly anisotropic thermoelectric responses in metals can be leveraged.
Methods
Ab initio calculations and group theoretical analysis
We perform first-principles density functional calculations within the local spin density approximation, which is known to provide a reliable description of perovskite ruthenates35,39,50 as implemented in the Vienna Ab initio Simulation Package51,52. Calculations carried out with the Perdew–Burke–Ernzerhof generalized gradient approximation functional revised for solids53 provide essentially the same results (see Supplementary Table 6). We use the projector augmented wave method54 to treat the core and valence electrons using a 550 eV plane wave expansion and the following electronic configurations: 4s24p65s2 (Sr), 3p64s2 (Ca), 4p65s24d6 (Ru) and 2s22p4 (O). A 7 × 7 × 5 Monkhorst-Pack k-point mesh55 and Gaussian smearing (20 meV width) was used for the Brillouin zone (BZ) sampling and integrations.
For structure optimization, we relax the atomic positions to have forces less than 0.1 meV Å−1. We impose as a constraint b=c (note that in bulk ruthenates the deviations between the ‘short’ axes are small39) to simulate the situation under thin-film growth on a cubic substrate with a square lattice net. We search for the ground state structure by computing the phonon frequencies and eigenmodes of the high-symmetry centrosymmetric (Sr,Ca)Ru2O6 structure (space group P4/mmm), and then systematically ‘freeze-in’ linear combinations of the unstable modes, performing structural optimization on these candidate low-symmetry structures until the global minimum is obtained. The group theoretical analysis was aided with the ISODISTORT56 and AMPLIMODES57,58 software.
Thermoelectric properties
For the calculation of the Seebeck coefficient and other transport properties, we use Boltzmann transport theory59 within the constant scattering time (τ) approximation as implemented in the boltztrap code61. We perform a non-self-consistent band-structure calculation with a much denser sampling of 27 × 33 × 33 (4,046 k-points in the irreducible BZ; 29,403 k-points in the full BZ), using the equilibrium ground state structure. The Seebeck coefficients S are calculated independently from τ. The electrical conductivity, however, requires a known value of τ, and so we introduce an empirical parameter. Here, the relaxation time value τ=0.23 × 10−14 s is employed in all calculations, which we obtain from fitting the room-temperature conductivity62 σ~2.7 × 105 S m−1 of solid solution Sr0.5Ca0.5RuO3. We believe this estimate to provide a meaningful approximation based on our excellent agreement with other physical properties of the solid solution reported in the main text.
The thermoelectric properties at room temperature are calculated using the zero-Kelvin polar structure based on the fact that the high-temperature structural transitions away from orthorhombic symmetry in bulk CaRuO3 and SrRuO3 do not occur until 1,550 K63 and 685 K, respectively64. On the basis of our calculated magnetic Curie temperatures of ~50 K, we also compute all thermoelectric responses using a non-magnetic configuration.
Additional information
How to cite this article: Puggioni, D. et al. Designing a robustly metallic noncenstrosymmetric ruthenate oxide with large thermopower anisotropy. Nat. Commun. 5:3432 doi: 10.1038/ncomms4432 (2014).
References
Lines, M. E. & Glass, A. M. Principles and Applications of Ferroelectrics and Related Materials Clarendon (1977).
Zhong, W., Vanderbilt, D. & Rabe, K. M. Phase transitions in BaTiO3 from first principles. Phys. Rev. Lett. 73, 1861–1864 (1994).
Anderson, P. W. & Blount, E. I. Symmetry considerations on martensitic transformations: "ferroelectric" metals? Phys. Rev. Lett. 14, 217–219 (1965).
Lawson, A. C. & Zachariasen, W. H. Low temperature lattice transformation of HfV2 . Phys. Lett. A 38, 1 (1972).
Mineev, V. P. & Yoshioka, Y. Optical activity of noncentrosymmetric metals. Phys. Rev. B 81, 094525 (2010).
Edelstein, V. M. Features of light reflection off metals with destroyed mirror symmetry. Phys. Rev. B 83, 113109 (2011).
Edelstein, V. M. Magnetoelectric effect in polar superconductors. Phys. Rev. Lett. 75, 2004–2007 (1995).
Edelstein, V. M. Magnetoelectric effect in dirty superconductors with broken mirror symmetry. Phys. Rev. B 72, 172501 (2005).
Edelstein, V. M. The Ginzburg-Landau equation for superconductors of polar symmetry. J. Phys. Condens. Matter 8, 339–349 (1996).
Bauer, E. et al. Superconductivity in the complex metallic alloy β-Al3Mg2 . Phys. Rev. B 76, 014528 (2007).
Bauer, E. et al. Unconventional superconducting phase in the weakly correlated noncentrosymmetric Mo3Al2C compound. Phys. Rev. B 82, 064511 (2010).
Poeppelmeier, K. R. et al. New family of planar cuprate superconductors: effect of nonmagnetic chains and planes. Phys. C Supercond. 185-189, 525–526 (1991).
Vaughey, J. T. et al. Synthesis and structure of a new family of cuprate superconductors: LnSr2Cu2GaO7 . Chem. Mater. 3, 935–940 (1991).
Sergienko, I. A. et al. Metallic "ferroelectricity" in the Pyrochlore Cd2Re2O7 . Phys. Rev. Lett. 92, 065501 (2004).
Shi, Y. et al. A ferroelectric-like structural transition in a metal. Nat. Mater. 12, 1024–1027 (2013).
Kolodiazhnyi, T., Tachibana, M., Kawaji, H., Hwang, J. & Takayama-Muromachi, E. Persistence of ferroelectricity in BaTiO3 through the insulator-metal transition. Phys. Rev. Lett. 104, 147602 (2010).
Das, A. K. et al. Studies on temperature dependent semiconductor to metal transitions in ZnO thin films sparsely doped with Al. J. Appl. Phys. 112, 103706 (2012).
Cao, G., McCall, S., Shepard, M., Crow, J. E. & Guertin, R. P. Thermal, magnetic, and transport properties of single-crystal Sr1−xCaxRuO3 (0≲ x≲1.0). Phys. Rev. B 56, 321–329 (1997).
Wang, Y., Liu, X., Burton, J. D., Jaswal, S. S. & Tsymbal, E. Y. Ferroelectric instability under screened coulomb interactions. Phys. Rev. Lett. 109, 247601 (2012).
Benedek, N. A. & Fennie, C. J. Hybrid improper ferroelectricity: a mechanism for controllable polarization-magnetization coupling. Phys. Rev. Lett. 106, 107204 (2011).
Bousquet, E. et al. Improper ferroelectricity in perovskite oxide artificial superlattices. Nature 452, 732–736 (2008).
Howard, C. J. & Stokes, H. T. Group-theoretical analysis of octahedral tilting in perovskites. Acta Crystallogr. B 54, 782–789 (1998).
King, G. & Woodward, P. M. Cation ordering in perovskites. J. Mater. Chem. 20, 5785–5796 (2010).
Dachraoui, W. et al. Short-range layered a-site ordering in double perovskites NaLaBB′O6 (B=Mn, Fe; B′=Nb, Ta). Chem. Mater. 23, 2398–2406 (2011).
Zubko, P., Gariglio, S., Gabay, M., Ghosez, P. & Triscone, J.-M. Interface physics in complex oxide heterostructures. Annu. Rev. Condens. Matter Phys. 2, 141–165 (2011).
Rondinelli, J. M. & Fennie, C. J. Octahedral rotation-induced ferroelectricity in cation ordered perovskites. Adv. Mater. 24, 1961–1968 (2012).
Mulder, A. T., Benedek, N. A., Rondinelli, J. M. & Fennie, C. J. Turning ABO3 anti-ferroelectrics into ferroelectrics: design rules for practical rotation-driven ferroelectricity in double perovskites and A3B2O7 Ruddlesden-Popper compounds. Adv. Funct. Mater. 23, 4810–4820 (2013).
Woodward, P. M. Octahedral tilting in perovskites. II. Structure stabilizing forces. Acta Crystallogr. B 53, 44–66 (1997).
Kunz, Martin. & Brown, I. David. Out-of-center distortions around octahedrally coordinated d0 transition metals. J. Solid State Chem. 115, 395–406 (1995).
Kobayashi, H., Nagata, M., Kanno, R. & Kawamoto, Y. Structural characterization of the orthorhombic perovskites: [ARuO3 (A=Ca, Sr, La, Pr)]. Mater. Res. Bull. 29, 1271–1280 (1994).
Lampis, N., Franchini, C., Satta, G., Geddo-Lehmann, A. & Massidda, S. Electronic structure of PbFe1/2Ta1/2O3: crystallographic ordering and magnetic properties. Phys. Rev. B 69, 064412 (2004).
Anderson, P. W. New approach to the theory of superexchange interactions. Phys. Rev. 115, 2–13 (1959).
Wu, H., Hu, Z., Khomskii, D. I. & Tjeng, L. H. Insulating state and the importance of the spin-orbit coupling in Ca3CoRhO6 . Phys. Rev. B 75, 245118 (2007).
Maitra, T. & Valenti, R. Orbital Order in ZnV2O4 . Phys. Rev. Lett. 99, 126401 (2007).
Rondinelli, J. M., Caffrey, N. M., Sanvito, S. & Spaldin, N. A. Electronic properties of bulk and thin film SrRuO3: search for the metal-insulator transition. Phys. Rev. B 78, 155107 (2008).
Aroyo, M. I., Kirov, A., Capillas, C., Perez-Mato, J. M. & Wondratschek, H. Bilbao Crystallographic Server. II. Representations of crystallographic point groups and space groups. Acta Crystallogr. A 62, 115–128 (2006).
Orobengoa, D., Capillas, C., Aroyo, M. I. & Perez-Mato, J. M. AMPLIMODES: symmetry-mode analysis on the Bilbao Crystallographic Server. J. Appl. Crystallogr. 42, 820–833 (2009).
Lee, J. H. & Rabe, K. M. Epitaxial-strain-induced multiferroicity in SrMnO3 from first principles. Phys. Rev. Lett. 104, 207204 (2010).
Zayak, A. T., Huang, X., Neaton, J. B. & Rabe, K. M. Manipulating magnetic properties of SrRuO3 and CaRuO3 with epitaxial and uniaxial strains. Phys. Rev. B 77, 214410 (2008).
Garcia-Fernandez, P., Verissimo-Alves, M., Bilc, D. I., Ghosez, P. & Junquera, J. First-principles modeling of the thermoelectric properties of SrTiO3/SrRuO3 superlattices. Phys. Rev. B 86, 085305 (2012).
Lee, S., Bock, J. A., Trolier-McKinstry, S. & Randall, C. A. Ferroelectric-thermoelectricity and mott transition of ferroelectric oxides with high electronic conductivity. J. Eur. Ceram. Soc. 32, 3971–3988 (2012).
Xi, T. G., Fei, Y., Choy, C. L., Leung, W. P. & Shao, C. F. Specific heat and thermal diffusivity of strontium barium niobate (Sr1−xBaxNb2O6) single crystals. J. Eur. Ceram. Soc. 71, 170–173 (1992).
Kolodiazhnyi, T. et al. Thermoelectric power, Hall effect, and mobility of n-type BaTiO3 . Phys. Rev. B 68, 085205 (2003).
Snyder, G. J. & Toberer, E. S. Complex thermoelectric materials. Nat. Mater. 7, 105–114 (2008).
Kaiser, A. B. & Uher, C. in Studies of High Temperature Superconductors Vol. 7 (ed. Narlikar, A. V.) 353(Nova Science (1991).
Prettl, W., Zeuner, S. & Lengfellner, H. Fast thermoelectric response of normal state YBa2Cu3O7−δ films. Appl. Phys. Lett. 66, 1833–1835 (1995).
Ong, K. P., Singh, D. J. & Wu, P. Unusual transport and strongly anisotropic thermopower in PtCoO2 and PdCoO2 . Phys. Rev. Lett. 104, 176601 (2010).
Burkov, A. T., Heinrich, A. & Vedernikov, M. V. Anisotropic thermoelectric materials, properties and applications. AIP Conf. Proc. 316, 76–80 (1994).
Warusawithana, M. P. et al. A ferroelectric oxide made directly on silicon,". Science 324, 367–370 (2009).
Zayak, A. T., Huang, X., Neaton, J. B. & Rabe, K. M. Structural, electronic, and magnetic properties of SrRuO3 under epitaxial strain. Phys. Rev. B 74, 094104 (2006).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P. et al. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 100, 136406 (2008).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Campbell, B. J., Stokes, H. T., Tanner, D. E. & Hatch, D. M. ISODISPLACE: a web-based tool for exploring structural distortions. J. Appl. Crystallogr. 39, 607–614 (2006).
Orobengoa, D., Capillas, C., Aroyo, M. I. & Perez-Mato, J. M. AMPLIMODES: symmetry-mode analysis on the Bilbao Crystallographic Server. J. Appl. Crystallogr. 42, 820–833 (2009).
Perez-Mato, J. M., Orobengoa, D. & Aroyo, M. I. Mode crystallography of distorted structures. Acta Crystallogr. A 66, 558–590 (2010).
Ashcroft, N. W. & Mermin, N. D. Solid State Physics Thomson Learning, Inc. (1976).
Ziman, J. M. Principles of the Theory of Solids University Press (1972).
Madsen, G. K. H. & Singh, D. J. Boltztrap. a code for calculating band-structure dependent quantities. Comput. Phys. Commun. 175, 67–71 (2006).
Keawprak, N., Tu, R. & Goto, T. Thermoelectric properties of Ca1−xSrxRuO3 compounds prepared by spark plasma sintering. J. Alloys Compd. 523, 182–187 (2012).
Vashook, V. et al. Structural stability of conducting oxide CaRuO3 at high temperatures. Appl. Phys. Lett. 90, 251913 (2007).
Kennedy, B. J., Hunter, B. A. & Hester, J. R. Synchrotron X-ray diffraction reexamination of the sequence of high-temperature phases in SrRuO3 . Phys. Rev. B 65, 224103 (2002).
Halasyamani, P. S. & Poeppelmeier, K. R. Non-centrosymmetric oxides. Chem. Mater. 10, 2753–2769 (1998).
Acknowledgements
This work was supported by the Army Research Office under grant no. W911NF-12-1-0133. We acknowledge the High Performance Computing Modernization Program (HPCMP) of the DOD for providing computational resources that have contributed to the research results reported herein. We thank K.R. Poepplemeier and C.J. Fennie for insightful discussions.
Author information
Authors and Affiliations
Contributions
The study was planned, calculations carried out and the manuscript prepared by D.P. and J.M.R. Both authors discussed the results, wrote and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-4, Supplementary Tables 1-6, Supplementary Note 1 and Supplementary Reference (PDF 421 kb)
Rights and permissions
About this article
Cite this article
Puggioni, D., Rondinelli, J. Designing a robustly metallic noncenstrosymmetric ruthenate oxide with large thermopower anisotropy. Nat Commun 5, 3432 (2014). https://doi.org/10.1038/ncomms4432
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms4432
This article is cited by
-
A large modulation of electron-phonon coupling and an emergent superconducting dome in doped strong ferroelectrics
Nature Communications (2021)
-
Design of a multifunctional polar metal via first-principles high-throughput structure screening
Communications Materials (2020)
-
Charge transport in a polar metal
npj Quantum Materials (2019)
-
Interface-induced magnetic polar metal phase in complex oxides
Nature Communications (2019)
-
Evidence for the weakly coupled electron mechanism in an Anderson-Blount polar metal
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.