Abstract
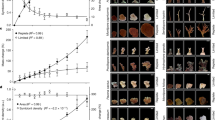
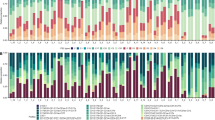
Rising ocean temperatures associated with global climate change are causing mass coral bleaching and mortality worldwide1. Understanding the genetic and environmental factors that mitigate coral bleaching susceptibility may aid local management efforts to help coral reefs survive climate change. Although bleaching susceptibility depends partly on the genetic identity of a coral’s algal symbionts2, the effect of symbiont density, and the factors controlling it, remain poorly understood. By applying a new metric of symbiont density3 to study the coral Pocillopora damicornis during seasonal warming and acute bleaching, we show that symbiont cell ratio density is a function of both symbiont type and environmental conditions, and that corals with high densities are more susceptible to bleaching. Higher vulnerability of corals with more symbionts establishes a quantitative mechanistic link between symbiont density and the molecular basis for coral bleaching, and indicates that high densities do not buffer corals from thermal stress, as has been previously suggested4. These results indicate that environmental conditions that increase symbiont densities, such as nutrient pollution5,6, will exacerbate climate-change-induced coral bleaching, providing a mechanistic explanation for why local management to reduce these stressors will help coral reefs survive future warming.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Baker, A. C., Glynn, P. W. & Riegl, B. Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 80, 435–471 (2008).
Glynn, P. W., Maté, J. L., Baker, A. C. & Calderón, M. Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Niño-Southern Oscillation event: Spatial/temporal patterns and comparisons with the 1982–1983 event. Bull. Mar. Sci. 69, 79–109 (2001).
Mieog, J. C., van Oppen, M. J. H., Berkelmans, R., Stam, W. T. & Olsen, J. L. Quantification of algal endosymbionts (Symbiodinium) in coral tissue using real-time PCR. Mol. Ecol. Resour. 9, 74–82 (2009).
Stimson, J., Sakai, K. & Sembali, H. Interspecific comparison of the symbiotic relationship in corals with high and low rates of bleaching-induced mortality. Coral Reefs 21, 409–421 (2002).
Falkowski, P. G., Dubinsky, Z., Muscatine, L. & McCloskey, L. Population control in symbiotic corals. Bioscience 43, 606–611 (1993).
Fabricius, K. E. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Poll. Bull. 50, 125–146 (2005).
Hoegh-Guldberg, O. et al. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (2007).
Baird, A. H., Bhagooli, R., Ralph, P. J. & Takahashi, S. Coral bleaching: The role of the host. Trends Ecol. Evol. 24, 16–20 (2009).
Brown, B. E., Dunne, R. P., Goodson, M. S. & Douglas, A. E. Bleaching patterns in reef corals. Nature 404, 142–143 (2000).
LaJeunesse, T. C. et al. Host–symbiont recombination versus natural selection in the response of coral–dinoflagellate symbioses to environmental disturbance. Proc. R. Soc. B 277, 2925–2934 (2010).
Iglesias-Prieto, R. & Trench, R. K. Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthetic unit to changes in photon flux density. Mar. Ecol. Prog. Ser. 113, 163–175 (1994).
Rowan, R. Coral bleaching—thermal adaptation in reef coral symbionts. Nature 430, 742–742 (2004).
Berkelmans, R. & van Oppen, M. J. H. The role of zooxanthellae in the thermal tolerance of corals: A ‘nugget of hope’ for coral reefs in an era of climate change. Proc. R. Soc. B 273, 2305–2312 (2006).
Muscatine, L. et al. Cell-specific density of symbiotic dinoflagellates in tropical anthozoans. Coral Reefs 17, 329–337 (1998).
Shick, J. M., Romaine-Lioud, S., Ferrier-Pagès, C. & Gattuso, J-P. Ultraviolet-B radiation stimulates shikimate pathway-dependent accumulation of mycosporine-like amino acids in the coral Stylophora pistillata despite decreases in its population of symbiotic dinoflagellates. Limnol. Oceanogr. 44, 1667–1682 (1999).
Fitt, W. K. et al. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol. Oceanogr. 45, 677–685 (2000).
Thornhill, D. J. et al. A connection between colony biomass and death in caribbean reef-building corals. PLoS ONE 6, e29535 (2011).
Ferrier-Pagès, C. et al. Summer autotrophy and winter heterotrophy in the temperate symbiotic coral Cladocora caespitosa. Limnol. Oceanogr. 56, 1429–1438 (2011).
Warner, M. E., Chilcoat, G. C., McFarland, F. K. & Fitt, W. K. Seasonal fluctuations in the photosynthetic capacity of photosystem II in symbiotic dinoflagellates in the Caribbean reef-building coral Montastraea. Mar. Biol. 141, 31–38 (2002).
Wooldridge, S. A. A new conceptual model for the warm-water breakdown of the coral-algae endosymbiosis. Mar. Fresh. Res. 60, 483–496 (2009).
McGuire, M. P. & Szmant, A. M. Time course of physiological responses to NH4 enrichment by a coral-zooxanthellae symbiosis. Proc. 8th Int. Coral Reef Symp. 1, 909–914 (1997).
Lesser, M. P. Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol. Oceanogr. 41, 271–283 (1996).
Downs, C. A. et al. Oxidative stress and seasonal coral bleaching. Free Radical Biol. Med. 33, 533–543 (2002).
Tchernov, D. et al. Apoptosis and the selective survival of host animals following thermal bleaching in zooxanthellate corals. Proc. Natl Acad. Sci. USA 108, 9905–9909 (2011).
Nesa, B. & Hidaka, M. High zooxanthella density shortens the survival time of coral cell aggregates under thermal stress. J. Exp. Mar. Biol. Ecol. 368, 81–87 (2009).
Wooldridge, S. A. A hypothesis linking sub-optimal seawater p CO 2 conditions for cnidarian-Symbiodinium symbioses with the exceedence of the interglacial threshold (>260 ppmv). Biogeosciences 9, 1709–1723 (2012).
Titlyanov, E. A., Titlyanova, T. V., Yamazato, K. & Van Woesik, R. Photo-acclimation dynamics of the coral Stylophora pistillata to low and extremely low light. J. Exp. Mar. Biol. Ecol. 263, 211–225 (2001).
Wooldridge, S. A. & Done, T. J. Improved water quality can ameliorate effects of climate change on corals. Ecol. Appl. 19, 1492–1499 (2009).
Rodolfo-Metalpa, R., Martin, S., Ferrier-Pagès, C. & Gattuso, J-P. Response of the temperate coral Cladocora caespitosa to mid- and long-term exposure to p CO 2 and temperature levels projected for the year 2100 AD. Biogeosciences 7, 481–481 (2010).
Wiedenmann, J. et al. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nature Clim. Change http://dx.doi.org/10.1038/nclimate1661 (in the press).
LaJeunesse, T. C. & Trench, R. K. Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal sea anemone Anthopleura elegantissima (Brandt). Biol. Bull. 199, 126–134 (2000).
Acknowledgements
We thank J. Maté and the Smithsonian Tropical Research Institute Naos Marine Laboratory for assistance with coral collections and export permits (ANAM SE/A-117-08, CITES SEX/A-140-08), the STRI Marine Environmental Program for the use of temperature data, M. Schmale for microscope use, C. Hurt and the University of Miami Molecular Core Facility for sequencing assistance, and J. Ault for statistical advice. Further support was provided by the National Science Foundation (OCE-0527184 to A.C.B. and OCE-0526361 to P. Glynn) and a Pew Fellowship in Marine Conservation to A.C.B. R.C. was supported by a University of Miami Fellowship and a National Science Foundation Graduate Research Fellowship.
Author information
Authors and Affiliations
Contributions
A.C.B. conceived the study and collected the corals. R.C. designed and validated the qPCR assays, conducted the repetitive sampling and analysed the data. The authors jointly interpreted the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 283 kb)
Rights and permissions
About this article
Cite this article
Cunning, R., Baker, A. Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nature Clim Change 3, 259–262 (2013). https://doi.org/10.1038/nclimate1711
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate1711
This article is cited by
-
Bio-optical signatures of in situ photosymbionts predict bleaching severity prior to thermal stress in the Caribbean coral species Acropora palmata
Coral Reefs (2024)
-
Meta-organism gene expression reveals that the impact of nitrate enrichment on coral larvae is mediated by their associated Symbiodiniaceae and prokaryotic assemblages
Microbiome (2023)
-
Viruses of a key coral symbiont exhibit temperature-driven productivity across a reefscape
ISME Communications (2023)
-
Coupled carbon and nitrogen cycling regulates the cnidarian–algal symbiosis
Nature Communications (2023)
-
The role of background algal symbionts as drivers of shuffling to thermotolerant Symbiodiniaceae following bleaching in three Caribbean coral species
Coral Reefs (2023)