Abstract
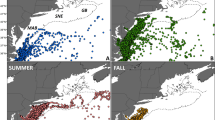
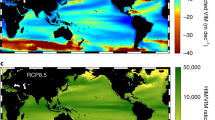
Climate model predictions1,2 and observations3,4 reveal regional declines in oceanic dissolved oxygen, which are probably influenced by global warming5. Studies indicate ongoing dissolved oxygen depletion and vertical expansion of the oxygen minimum zone (OMZ) in the tropical northeast Atlantic Ocean6,7. OMZ shoaling may restrict the usable habitat of billfishes and tunas to a narrow surface layer8,9. We report a decrease in the upper ocean layer exceeding 3.5 ml l−1 dissolved oxygen at a rate of ≤1 m yr−1 in the tropical northeast Atlantic (0–25° N, 12–30° W), amounting to an annual habitat loss of ∼5.95×1013 m3, or 15% for the period 1960–2010. Habitat compression and associated potential habitat loss was validated using electronic tagging data from 47 blue marlin. This phenomenon increases vulnerability to surface fishing gear for billfishes and tunas8,9, and may be associated with a 10–50% worldwide decline of pelagic predator diversity10. Further expansion of the Atlantic OMZ along with overfishing may threaten the sustainability of these valuable pelagic fisheries and marine ecosystems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bopp, L., Le Quere, C., Heimann, M., Manning, A. C. & Monfray, P. Climate induced oceanic oxygen fluxes: Implications for the contemporary carbon budget. Glob. Biogeochem. Cycles 16, 1022 (2002).
Oschlies, A., Schultz, K. G., Riebesell, U. & Schmittner, A. Simulated 21st century’s increase in oceanic suboxia in CO2-enhanced biotic carbon export. Glob. Biogeochem. Cycles 22, GB4008 (2008).
Stramma, L., Johnson, G. C., Sprintall, J. & Mohrholz, V. Expanding oxygen-minimum zones in the tropical oceans. Science 320, 655–658 (2008).
Bograd, S. J. et al. Oxygen declines and the shoaling of the hypoxic boundary in the California Current. Geophys. Res. Lett. 35, L12607 (2008).
Keeling, R. F., Körtzinger, A. & Gruber, N. Ocean deoxygenation in a warming world. Annu. Rev. Mar. Sci. 2, 199–229 (2010).
Stramma, L. et al. Oxygen minimum zone in the North Atlantic south and east of the Cape Verde Islands. J. Geophys. Res. 113, C04014 (2008).
Stramma, L., Visbeck, M., Brandt, P., Tanhua, T. & Wallace, D. Deoxygenation in the oxygen minimum zone of the eastern tropical North Atlantic. Geophys. Res. Lett. 36, L20607 (2009).
Prince, E. D. & Goodyear, C. P. Hypoxia-based habitat compression of tropical pelagic fishes. Fish. Oceanogr. 15, 451–464 (2006).
Prince, E. D. et al. Ocean scale hypoxia-based habitat compression of Atlantic Istiophorid billfishes. Fish. Oceangr. 19, 448–462 (2010).
Worm, B., Sandow, M., Oschlies, A., Lotze, H. K. & Myers, R. A. Global patterns of predator diversity in the open oceans. Science 308, 1365–1369 (2005).
Chan, F. et al. Emergence of anoxia in the California Current large marine ecosystem. Science 319, 920 (2008).
Diaz, R. J. & Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 321, 926–929 (2008).
Randell, D. J. Fish Physiology. The Nervous System. Circulation, and Respiration 253–292 (Academic, 1970).
Whitney, F. A., Freeland, H. J. & Robert, M. Persistently declining oxygen levels in the interior waters of the eastern subarctic Pacific. Prog. Oceanogr. 75, 179–199 (2007).
Brill, R. W. A review of temperature and oxygen tolerance studies of tunas pertinent to fisheries oceanography, movement models and stock assessments. Fish. Oceanogr. 3, 204–216 (1994).
Roberts, J. L. The Physiological Ecology of Tunas 83–88 (Academic, 1978).
Brill, R. W. Selective advantages conferred by the high performance physiology of tunas, billfish, and dolphin fish. Comp. Biochem. Physiol. 113, 3–15 (1996).
Seibel, B. A. Critical oxygen levels and metabolic suppression in oceanic oxygen minimum zones. J. Exp. Biol 214, 326–336 (2011).
Idrisi, N. et al. Behavior, oxygen consumption and survival of stressed sailfish (Istiophorus platypterus) in captivity. Mar. Fresh. Behav. Phys. Water. 36, 51–57 (2002).
Ekau, W., Auel, H., Portner, H-O. & Gilbert, D. Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeoscience 7, 1669–1699 (2010).
Wegner, N. C., Sepulveda, C. A., Bull, K. B. & Graham, J. B. Gill morphometrics in relation to gas transfer and ram ventilation in high-energy demand teleosts: Scombrids and billfishes. J. Morphol. 271, 36–49 (2010).
International Commission for Conservation of Atlantic Tunas Report for Biennial Period 2010–2011, Part I Vol. 2, 12–118 (ICCAT, 2011).
Domingues, C. M. et al. Improved estimates of upper-ocean warming and multi-decadal sea-level rise. Nature 453, 1090–1093 (2008).
Brandt, P. et al. Changes in the ventilation of the oxygen minimum zone of the tropical North Atlantic. J. Phys. Oceanogr. 40, 1784–1801 (2010).
Kreiner, A., Stenevik, E. K. & Ekau, W. Sardine Sardinops sagax and anchovy Engraulis encrasicolus larvae avoid regions with low dissolved oxygen concentrations in the northern Benguela Current system. J. Fish. Biol. 74, 270–277 (2009).
Goodyear, C. P. et al. Vertical habitat use of Atlantic blue marlin Makaira nigricans: Interaction with pelagic longline gear. Mar. Ecol. Prog. Ser. 365, 233–245 (2008).
Cushing, D. Upwelling and Fish Production Vol. 84 (Fish. Tech. Paper, Fishery Resources and Exploitation Division, Food and Agricultural Organization of the United Nations, 1969).
Lowe, T., Brill, R. & Cousins, K. Blood oxygen-binding characteristics of bigeye tuna (Thunnus obesus), a high-energy-demand teleost that is tolerant of low ambient oxygen. Mar. Biol. 136, 1087–1098 (2000).
Gilly, W. F. et al. Vertical and horizontal migrations by the jumbo squid Dosidicus gigas revealed by electronic tagging. Mar. Ecol. Prog. Ser 324, 1–17 (2006).
Worm, B. et al. Rebuilding global fisheries. Science 325, 578–585 (2009).
Brewer, P. G. & Peltzer, E. T. Limits to marine life. Science 324, 347–348 (2009).
Acknowledgements
The Deutsche Forschungsgemeinschaft (DFG) provided support as part of the Collaborative Research Center SFB-754 (L.S., M.V., D.W.R.W., P.B. and A.K.). Support for the biological part of the study was provided through the Southeast Fisheries Science Center, The Billfish Foundation and the Adopt-A-Billfish Program (E.D.P.). Additional support was provided through the NOAA Climate Program Office and the NOAA Office of Oceanic and Atmospheric Research (S.S.). Support for J.L. and J.P.H. was provided by the Cooperative Institute for Marine and Atmospheric Studies (CIMAS), a Cooperative Institute of the University of Miami and the National Oceanic and Atmospheric Administration, cooperative agreement NA1RJ1226.
Author information
Authors and Affiliations
Contributions
L.S., E.D.P. and S.S. designed the experiment. E.D.P. and J.P.H. contributed biological expertise and biological data sets. S.S. and J.L. carried out the oceanographic and biological computations and did the art work. M.V., D.W.R.W., P.B. and A.K. contributed data and Atlantic Ocean expertise. E.D.P., L.S., J.P.H. and S.S. wrote the paper. All authors discussed the results and commented on the manuscript. L.S., E.D.P. and S.S. are equally contributing first authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Stramma, L., Prince, E., Schmidtko, S. et al. Expansion of oxygen minimum zones may reduce available habitat for tropical pelagic fishes. Nature Clim Change 2, 33–37 (2012). https://doi.org/10.1038/nclimate1304
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate1304
This article is cited by
-
Hydrological cycle amplification reshapes warming-driven oxygen loss in the Atlantic Ocean
Nature Climate Change (2024)
-
Highly active fish in low oxygen environments: vertical movements and behavioural responses of bigeye and yellowfin tunas to oxygen minimum zones in the eastern Pacific Ocean
Marine Biology (2024)
-
Intermediate water circulation drives distribution of Pliocene Oxygen Minimum Zones
Nature Communications (2023)
-
MArVD2: a machine learning enhanced tool to discriminate between archaeal and bacterial viruses in viral datasets
ISME Communications (2023)
-
Reconstructing consequences of lifetime hypoxia exposure on metabolism of demersal fish in the northern Gulf of Mexico using otolith chemistry
Environmental Biology of Fishes (2023)