Abstract
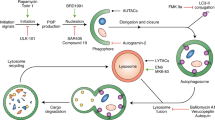
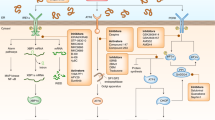
Chaperone-mediated autophagy (CMA) contributes to cellular quality control and the cellular response to stress through the selective degradation of cytosolic proteins in lysosomes. A decrease in CMA activity occurs in aging and in age-related disorders (for example, neurodegenerative diseases and diabetes). Although prevention of this age-dependent decline through genetic manipulation in mice has proven beneficial, chemical modulation of CMA is not currently possible, owing in part to the lack of information on the signaling mechanisms that modulate this pathway. In this work, we report that signaling through retinoic acid receptor α (RARα) inhibits CMA and apply structure-based chemical design to develop synthetic derivatives of all-trans-retinoic acid to specifically neutralize this inhibitory effect. We demonstrate that chemical enhancement of CMA protects cells from oxidative stress and from proteotoxicity, supporting a potential therapeutic opportunity when reduced CMA contributes to cellular dysfunction and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
Change history
28 June 2013
In the version of this article initially published, one of the three gray bars in Figure 6a was not defined, and the asterisks for these bars were misaligned. The errors have been corrected in the HTML and PDF versions of the article.
References
Mizushima, N., Levine, B., Cuervo, A.M. & Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008).
Yang, Z. & Klionsky, D.J. An overview of the molecular mechanism of autophagy. Curr. Top. Microbiol. Immunol. 335, 1–32 (2009).
Mizushima, N. Autophagy in protein and organelle turnover. Cold Spring Harb. Symp. Quant. Biol. 76, 397–402 (2011).
Wong, E. & Cuervo, A.M. Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 13, 805–811 (2010).
Arias, E. & Cuervo, A.M. Chaperone-mediated autophagy in protein quality control. Curr. Opin. Cell Biol. 23, 184–189 (2011).
Dice, J.F. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem. Sci. 15, 305–309 (1990).
Chiang, H.L., Terlecky, S., Plant, C. & Dice, J.F. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science 246, 382–385 (1989).
Bandyopadhyay, U., Kaushik, S., Varticovski, L. & Cuervo, A.M. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol. Cell Biol. 28, 5747–5763 (2008).
Cuervo, A.M., Stefanis, L., Fredenburg, R., Lansbury, P.T. & Sulzer, D. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295 (2004).
Mak, S.K., McCormack, A.L., Manning-Bog, A.B., Cuervo, A.M. & Di Monte, D.A. Lysosomal degradation of α-synuclein in vivo. J. Biol. Chem. 285, 13621–13629 (2010).
Wang, Y. et al. Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum. Mol. Genet. 18, 4153–4170 (2009).
Sooparb, S., Price, S.R., Shaoguang, J. & Franch, H.A. Suppression of chaperone-mediated autophagy in the renal cortex during acute diabetes mellitus. Kidney Int. 65, 2135–2144 (2004).
Venugopal, B. et al. Chaperone-mediated autophagy is defective in mucolipidosis type IV. J. Cell. Physiol. 219, 344–353 (2009).
Cuervo, A.M. & Dice, J.F. Age-related decline in chaperone-mediated autophagy. J. Biol. Chem. 275, 31505–31513 (2000).
Zhang, C. & Cuervo, A.M. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat. Med. 14, 959–965 (2008).
Finn, P.F., Mesires, N., Vine, M. & Dice, J.F. Effects of small molecules on chaperone-mediated autophagy. Autophagy 1, 141–145 (2005).
Duong, V. & Rochette-Egly, C. The molecular physiology of nuclear retinoic acid receptors. From health to disease. Biochim. Biophys. Acta 1812, 1023–1031 (2011).
Kon, M. et al. Chaperone-mediated autophagy is required for tumor growth. Sci. Transl. Med. 3, 109ra117 (2011).
Frolik, C.A., Roller, P.P., Roberts, A.B. & Sporn, M.B. In vitro and in vivo metabolism of all-trans- and 13-cis-retinoic acid in hamsters. Identification of 13-cis-4-oxoretinoic acid. J. Biol. Chem. 255, 8057–8062 (1980).
Rochette-Egly, C. & Germain, P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs). Nucl. Recept. Signal. 7, e005 (2009).
de Lera, A.R., Bourguet, W., Altucci, L. & Gronemeyer, H. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat. Rev. Drug Discov. 6, 811–820 (2007).
Njar, V.C. et al. Retinoic acid metabolism blocking agents (RAMBAs) for treatment of cancer and dermatological diseases. Bioorg. Med. Chem. 14, 4323–4340 (2006).
Das, B.C. et al. Design and synthesis of 3,5-disubstituted 1,2,4-oxadiazole containing retinoids from a retinoic acid receptor agonist. Tetrahedr. Lett. 52, 2433–2435 (2011).
Das, B.C., McCartin, K., Liu, T.C., Peterson, R.T. & Evans, T. A forward chemical screen in zebrafish identifies a retinoic acid derivative with receptor specificity. PLoS ONE 5, e10004 (2010).
Isakson, P., Bjoras, M., Boe, S.O. & Simonsen, A. Autophagy contributes to therapy-induced degradation of the PML/RARA oncoprotein. Blood 116, 2324–2331 (2010).
Wang, Z. et al. Autophagy regulates myeloid cell differentiation by p62/SQSTM1–mediated degradation of PML-RARα oncoprotein. Autophagy 7, 401–411 (2011).
Trocoli, A. et al. ATRA-induced upregulation of Beclin 1 prolongs the life span of differentiated acute promyelocytic leukemia cells. Autophagy 7, 1108–1114 (2011).
Rajawat, Y., Hilioti, Z. & Bossis, I. Retinoic acid induces autophagosome maturation through redistribution of the cation-independent mannose-6-phosphate receptor. Antioxid. Redox Signal. 14, 2165–2177 (2011).
Tanida, I., Minematsu-Ikeguchi, N., Ueno, T. & Kominami, E. Lysosomal turnover, but not a cellular level, of endogenous lc3 is a marker for autophagy. Autophagy 1, 84–91 (2005).
Koga, H., Martinez-Vicente, M., Verkhusha, V.V. & Cuervo, A.M. A photoconvertible fluorescent reporter to track chaperone-mediated autophagy. Nat. Commun. 2, 386 (2011).
Das, B.C., Anguiano, J. & Mahalingam, S.M. Design and synthesis of α-aminonitrile–functionalized novel retinoids. Tetrahedr. Lett. 50, 5670–5672 (2009).
Das, B.C. et al. Design and synthesis of potential new apoptosis agents: hybrid compounds containing perillyl alcohol and new constrained retinoids. Tetrahedr. Lett. 51, 1462–1466 (2010).
Das, B.C., Madhukumar, A.V., Anguiano, J. & Mani, S. Design, synthesis and biological evaluation of 2H-benzo[b][1,4] oxazine derivatives as hypoxia targeted compounds for cancer therapeutics. Bioorg. Med. Chem. Lett. 19, 4204–4206 (2009).
Massey, A.C., Kaushik, S., Sovak, G., Kiffin, R. & Cuervo, A.M. Consequences of the selective blockage of chaperone-mediated autophagy. Proc. Natl. Acad. Sci. USA 103, 5805–5810 (2006).
Ahlemeyer, B. et al. Retinoic acid reduces apoptosis and oxidative stress by preservation of SOD protein level. Free Radic. Biol. Med. 30, 1067–1077 (2001).
Kiffin, R., Christian, C., Knecht, E. & Cuervo, A. Activation of chaperone-mediated autophagy during oxidative stress. Mol. Biol. Cell 15, 4829–4840 (2004).
Eskelinen, E.L. et al. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol. Biol. Cell 13, 3355–3368 (2002).
Sardiello, M. et al. A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009).
Delacroix, L. et al. Cell-specific interaction of retinoic acid receptors with target genes in mouse embryonic fibroblasts and embryonic stem cells. Mol. Cell Biol. 30, 231–244 (2010).
Kaushik, S. & Cuervo, A.M. Methods to monitor chaperone-mediated autophagy. Methods Enzymol. 452, 297–324 (2009).
Klionsky, D.J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8, 445–544 (2012).
Cuervo, A.M., Dice, J.F. & Knecht, E. A population of rat liver lysosomes responsible for the selective uptake and degradation of cytosolic proteins. J. Biol. Chem. 272, 5606–5615 (1997).
Shridhar, D.R., Reddy, C.V., Sastry, O.P., Bansal, O.P. & Rao, P.P. A convenient one-step synthesis of 3-aryl-2H–1,4-benzoxazines. Synthesis 1981, 912–913 (1981).
Friesner, R.A. et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 49, 6177–6196 (2006).
Halgren, T.A. et al. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 47, 1750–1759 (2004).
Friesner, R.A. et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 47, 1739–1749 (2004).
Shivakumar, D. et al. Prediction of Absolute solvation free energies using molecular dynamics free energy perturbation and the OPLS force field. J. Chem. Theory Comput. 6, 1509–1519 (2010).
Guo, Z. et al. Probing the α-helical structural stability of stapled p53 peptides: molecular dynamics simulations and analysis. Chem. Biol. Drug Des. 75, 348–359 (2010).
Bowers, K.J., Dror, R.O. & Shaw, D.E. The midpoint method for parallelization of particle simulations. J. Chem. Phys. 124, 184109 (2006).
Acknowledgements
We thank R. Valdor for technical assistance with the luciferase assay, R. Kiffin for assistance with the quantitative RT-PCR, F. Macian for help with fluorescence-activated cell sorting procedures, T. Evans and I. Torregroza for advice with the RARα luciferase assay, C.-L. Towse for advice on the simulated annealing and molecular dynamics simulations and S. Kaushik for critically reviewing this manuscript. This work was supported by grants from the US National Institutes of Health (NIH)–National Institute on Aging (AG021904 and AG031782 to A.M.C.); Albert Einstein College of Medicine start-up funds (to E.G.); NIH–National Heart, Lung, and Blood Institute (HL095929 to E.G.); NIH–National Institute on Alcohol Abuse and Alcoholism (AA020630 to B.C.D.); and by the Rainwaters Foundation, the Beatrice and Roy Backus Foundation and a Robert and Renee Belfer gift (to A.M.C.).
Author information
Authors and Affiliations
Contributions
J.A. performed the experiments, analyzed the data and contributed to writing the paper; T.P.G. performed the in silico docking and molecular dynamics simulations; M.M. contributed to the synthesis of chemical compounds; B.C.D. designed the chemical compounds, analyzed the chemical data and revised the manuscript; E.G. designed and directed the in silico docking and molecular dynamics simulations and contributed to the interpretation of the chemical data and to the writing and revising of the manuscript; and A.M.C. designed the biological experiments, directed the study and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results (PDF 2544 kb)
Supplementary Note
Supplementary Note 1 (PDF 72 kb)
Rights and permissions
About this article
Cite this article
Anguiano, J., Garner, T., Mahalingam, M. et al. Chemical modulation of chaperone-mediated autophagy by retinoic acid derivatives. Nat Chem Biol 9, 374–382 (2013). https://doi.org/10.1038/nchembio.1230
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1230
This article is cited by
-
Chaperone-mediated autophagy in neurodegenerative diseases: mechanisms and therapy
Molecular and Cellular Biochemistry (2023)
-
Targeting retinoic acid receptor alpha-corepressor interaction activates chaperone-mediated autophagy and protects against retinal degeneration
Nature Communications (2022)
-
Lysosomal lipid alterations caused by glucocerebrosidase deficiency promote lysosomal dysfunction, chaperone-mediated-autophagy deficiency, and alpha-synuclein pathology
npj Parkinson's Disease (2022)
-
The biological clean-ups that could combat age-related disease
Nature (2022)
-
Mitophagy in neurological disorders
Journal of Neuroinflammation (2021)