Abstract
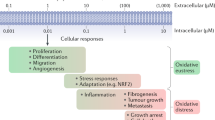
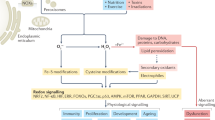
Reactive oxygen species (ROS) are a family of molecules that are continuously generated, transformed and consumed in all living organisms as a consequence of aerobic life. The traditional view of these reactive oxygen metabolites is one of oxidative stress and damage that leads to decline of tissue and organ systems in aging and disease. However, emerging data show that ROS produced in certain situations can also contribute to physiology and increased fitness. This Perspective provides a focused discussion on what factors lead ROS molecules to become signal and/or stress agents, highlighting how increasing knowledge of the underlying chemistry of ROS can lead to advances in understanding their disparate contributions to biology. An important facet of this emerging area at the chemistry-biology interface is the development of new tools to study these small molecules and their reactivity in complex biological systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Blake, C.C. et al. Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution. Nature 206, 757–761 (1965).
Barnham, K.J., Masters, C.L. & Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 3, 205–214 (2004).
Finkel, T., Serrano, M. & Blasco, M.A. The common biology of cancer and ageing. Nature 448, 767–774 (2007).
Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181–189 (2004).
Rhee, S.G. H2O2, a necessary evil for cell signaling. Science 312, 1882–1883 (2006).
Stone, J.R. & Yang, S. Hydrogen peroxide: a signaling messenger. Antioxid. Redox Signal. 8, 243–270 (2006).
Veal, E.A., Day, A.M. & Morgan, B.A. Hydrogen peroxide sensing and signaling. Mol. Cell 26, 1–14 (2007).
D'Autréaux, B. & Toledano, M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 (2007).
Paulsen, C.E. & Carroll, K.S. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem. Biol. 5, 47–62 (2010).
Cochemé, H.M. et al. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 13, 340–350 (2011).
Ignarro, L.J. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J. Physiol. Pharmacol. 53, 503–514 (2002).
Bryan, N.S., Bian, K. & Murad, F. Discovery of the nitric oxide signaling pathway and targets for drug development. Front. Biosci. 14, 1–18 (2009).
Szabó, C., Ischiropoulos, H. & Radi, R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 6, 662–680 (2007).
Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 4, 278–286 (2008).
del Río, L.A., Sandalio, L.M., Palma, J.M., Bueno, P. & Corpas, F.J. Metabolism of oxygen radicals in peroxisomes and cellular implications. Free Radic. Biol. Med. 13, 557–580 (1992).
Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 (2009).
Schriner, S.E. et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005).
Lee, H.Y. et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 12, 668–674 (2010).
Avshalumov, M.V. & Rice, M.E. Activation of ATP-sensitive K+ (KATP) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proc. Natl. Acad. Sci. USA 100, 11729–11734 (2003).
Bao, L. et al. Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signaling. J. Neurosci. 29, 9002–9010 (2009).
Giorgio, M. et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122, 221–233 (2005).
Migliaccio, E. et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402, 309–313 (1999).
Veeramani, S., Yuan, T.C., Lin, F.F. & Lin, M.F. Mitochondrial redox signaling by p66Shc is involved in regulating androgenic growth stimulation of human prostate cancer cells. Oncogene 27, 5057–5068 (2008).
Gross, E. et al. Generating disulfides enzymatically: reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc. Natl. Acad. Sci. USA 103, 299–304 (2006).
Chen, K., Kirber, M.T., Xiao, H., Yang, Y. & Keaney, J.F. Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 181, 1129–1139 (2008).
Suh, Y.A. et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401, 79–82 (1999).
Geiszt, M., Kopp, J.B., Várnai, P. & Leto, T.L. Identification of Renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. USA 97, 8010–8014 (2000).
Bedard, K. & Krause, K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313 (2007).
Sundaresan, M., Yu, Z.X., Ferrans, V.J., Irani, K. & Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270, 296–299 (1995).
Peskin, A.V. et al. The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants and thiol reagents. J. Biol. Chem. 282, 11885–11892 (2007).
Denu, J.M. & Tanner, K.G. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry 37, 5633–5642 (1998).
Salmeen, A. et al. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 423, 769–773 (2003).
van Montfort, R.L., Congreve, M., Tisi, D., Carr, R. & Jhoti, H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature 423, 773–777 (2003).
Biteau, B., Labarre, J. & Toledano, M.B. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 425, 980–984 (2003).
Chang, T.S. et al. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J. Biol. Chem. 279, 50994–51001 (2004).
Kwon, J. et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. USA 101, 16419–16424 (2004).
Wood, M.J., Storz, G. & Tjandra, N. Structural basis for redox regulation of Yap1 transcription factor localization. Nature 430, 917–921 (2004).
Dansen, T.B. et al. Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat. Chem. Biol. 5, 664–672 (2009).
Fu, X., Kassim, S.Y., Parks, W.C. & Heinecke, J.W. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J. Biol. Chem. 276, 41279–41287 (2001).
Ago, T. et al. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell 133, 978–993 (2008).
Stadtman, E.R. Protein oxidation and aging. Free Radic. Res. 40, 1250–1258 (2006).
Winterbourn, C.C. & Kettle, A.J. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic. Biol. Med. 29, 403–409 (2000).
Shao, B., Oda, M.N., Oram, J.F. & Heinecke, J.W. Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein. Chem. Res. Toxicol. 23, 447–454 (2010).
Thomson, E. et al. Identifying peroxidases and their oxidants in the early pathology of cystic fibrosis. Free Radic. Biol. Med. 49, 1354–1360 (2010).
Moskovitz, J. et al. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc. Natl. Acad. Sci. USA 98, 12920–12925 (2001).
Lee, J.W. & Helmann, J.D. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature 440, 363–367 (2006).
Gaudu, P. & Weiss, B. SoxR, a [2Fe-2S] transcription factor, is active only in its oxidized form. Proc. Natl. Acad. Sci. USA 93, 10094–10098 (1996).
Hilenski, L.L., Clempus, R.E., Quinn, M.T., Lambeth, J.D. & Griendling, K.K. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 24, 677–683 (2004).
Ushio-Fukai, M. Localizing NADPH oxidase–derived ROS. Sci. STKE 2006, re8 (2006).
Mishina, N.M. et al. Does cellular hydrogen peroxide diffuse or act locally? Antioxid. Redox Signal. 14, 1–7 (2011).
Wu, R.F., Ma, Z., Liu, Z. & Terada, L.S. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol. Cell. Biol. 30, 3553–3568 (2010).
Wood, Z.A., Poole, L.B. & Karplus, P.A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300, 650–653 (2003).
Woo, H.A. et al. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science 300, 653–656 (2003).
Woo, H.A. et al. Inactivation of Peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell 140, 517–528 (2010).
Bienert, G.P. et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282, 1183–1192 (2007).
Dynowski, M., Schaaf, G., Loque, D., Moran, O. & Ludewig, U. Plant plasma membrane water channels conduct the signalling molecule H2O2 . Biochem. J. 414, 53–61 (2008).
Miller, E.W., Dickinson, B.C. & Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 107, 15681–15686 (2010).
Kim, J.S., Huang, T.Y. & Bokoch, G.M. Reactive oxygen species regulate a slingshot-cofilin activation pathway. Mol. Biol. Cell 20, 2650–2660 (2009).
Gianni, D., Taulet, N., DerMardirossian, C. & Bokoch, G.M. c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol. Biol. Cell 21, 4287–4298 (2010).
Niethammer, P., Grabher, C., Look, A.T. & Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459, 996–999 (2009).
O'Neill, J.S. & Reddy, A.B. Circadian clocks in human red blood cells. Nature 469, 498–503 (2011).
O'Neill, J.S. et al. Circadian rhythms persist without transcription in a eukaryote. Nature 469, 554–558 (2011).
Dickinson, B.C., Peltier, J., Stone, D., Schaffer, D.V. & Chang, C.J. Nox2 redox signaling maintains essential cell populations in the brain. Nat. Chem. Biol. 7, 106–112 (2011).
Le Belle, J.E. et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 8, 59–71 (2011).
Hempel, S.L., Buettner, G.R., O'Malley, Y.Q., Wessels, D.A. & Flaherty, D.M. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic. Biol. Med. 27, 146–159 (1999).
Ostergaard, H., Henriksen, A., Hansen, F.G. & Winther, J.R. Shedding light on disulfide bond formation: engineering a redox switch in green fluorescent protein. EMBO J. 20, 5853–5862 (2001).
Hanson, G.T. et al. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J. Biol. Chem. 279, 13044–13053 (2004).
Sasaki, E. et al. Highly sensitive near-infrared fluorescent probes for nitric oxide and their application to isolated organs. J. Am. Chem. Soc. 127, 3684–3685 (2005).
Lim, M.H., Xu, D. & Lippard, S.J. Visualization of nitric oxide in living cells by a copper-based fluorescent probe. Nat. Chem. Biol. 2, 375–380 (2006).
Yang, D., Wang, H.L., Sun, Z.N., Chung, N.W. & Shen, J.G. A highly selective fluorescent probe for the detection and imaging of peroxynitrite in living cells. J. Am. Chem. Soc. 128, 6004–6005 (2006).
Robinson, K.M. et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. USA 103, 15038–15043 (2006).
Kenmoku, S., Urano, Y., Kojima, H. & Nagano, T. Development of a highly specific rhodamine-based fluorescence probe for hypochlorous acid and its application to real-time imaging of phagocytosis. J. Am. Chem. Soc. 129, 7313–7318 (2007).
Belousov, V.V. et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 3, 281–286 (2006).
Miller, E.W., Bian, S.X. & Chang, C.J. A fluorescent sensor for imaging reversible redox cycles in living cells. J. Am. Chem. Soc. 129, 3458–3459 (2007).
Robinson, K.M., Janes, M.S. & Beckman, J.S. The selective detection of mitochondrial superoxide by live cell imaging. Nat. Protoc. 3, 941–947 2008).
Gutscher, M. et al. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 5, 553–559 (2008).
Wang, W. et al. Superoxide flashes in single mitochondria. Cell 134, 279–290 (2008).
Garner, A.L. et al. Specific fluorogenic probes for ozone in biological and atmospheric samples. Nature Chemistry 1, 316–321 (2009).
Dickinson, B.C., Srikun, D. & Chang, C.J. Mitochondrial-targeted fluorescent probes for reactive oxygen species. Curr. Opin. Chem. Biol. 14, 50–56 (2010).
Chang, M.C., Pralle, A., Isacoff, E.Y. & Chang, C.J. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J. Am. Chem. Soc. 126, 15392–15393 (2004).
Miller, E.W., Albers, A.E., Pralle, A., Isacoff, E.Y. & Chang, C.J. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J. Am. Chem. Soc. 127, 16652–16659 (2005).
Miller, E.W., Tulyanthan, O., Isacoff, E.Y. & Chang, C.J. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat. Chem. Biol. 3, 263–267 (2007).
Dickinson, B.C. & Chang, C.J. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J. Am. Chem. Soc. 130, 9638–9639 (2008).
Srikun, D., Miller, E.W., Domaille, D.W. & Chang, C.J. An ICT-based approach to ratiometric fluorescence imaging of hydrogen peroxide produced in living cells. J. Am. Chem. Soc. 130, 4596–4597 (2008).
Dickinson, B.C., Huynh, C. & Chang, C.J. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J. Am. Chem. Soc. 132, 5906–5915 (2010).
Lippert, A.R., Keshari, K.R., Kurhanewicz, J. & Chang, C.J. A hydrogen peroxide-responsive hyperpolarized (13)c MRI contrast agent. J. Am. Chem. Soc. 133, 3776–3779 (2011).
Srikun, D., Albers, A.E. & Chang, C.J. A dendrimer-based platform for simultaneous dual fluorescence imaging of hydrogen peroxide and pH gradients produced in living cells. Chem. Sci. 2, 1156–1165 (2011).
Lee, D. et al. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 6, 765–769 (2007).
Van de Bittner, G.C., Dubikovskaya, E.A., Bertozzi, C.R. & Chang, C.J. In vivo imaging of hydrogen peroxide production in a murine tumor model with a chemoselective bioluminescent reporter. Proc. Natl. Acad. Sci. USA 107, 21316–21321 (2010).
Haskew-Layton, R.E. et al. Controlled enzymatic production of astrocytic hydrogen peroxide protects neurons from oxidative stress via an Nrf2-independent pathway. Proc. Natl. Acad. Sci. USA 107, 17385–17390 (2010).
Mahon, K.P. et al. Deconvolution of the cellular oxidative stress response with organelle-specific peptide conjugates. Chem. Biol. 14, 923–930 (2007).
Miller, E.W. et al. Light-activated regulation of cofilin dynamics using a photocaged hydrogen peroxide generator. J. Am. Chem. Soc. published online, doi: 10.1021/ja107783j (15 November 2010).
Winter, J., Ilbert, M., Graf, P.C., Ozcelik, D. & Jakob, U. Bleach activates a redox-regulated chaperone by oxidative protein unfolding. Cell 135, 691–701 (2008).
Leichert, L.I. et al. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc. Natl. Acad. Sci. USA 105, 8197–8202 (2008).
Fomenko, D.E., Xing, W., Adair, B.M., Thomas, D.J. & Gladyshev, V.N. High-throughput identification of catalytic redox-active cysteine residues. Science 315, 387–389 (2007).
Weerapana, E. et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 (2010).
Poole, L.B. et al. Fluorescent and affinity-based tools to detect cysteine sulfenic acid formation in proteins. Bioconjug. Chem. 18, 2004–2017 (2007).
Leonard, S.E., Reddie, K.G. & Carroll, K.S. Mining the thiol proteome for sulfenic acid modifications reveals new targets for oxidation in cells. ACS Chem. Biol. 4, 783–799 (2009).
Seo, Y.H. & Carroll, K.S. Quantification of protein sulfenic acid modifications using isotope-coded dimedone and iododimedone. Angew. Chem. Int. Edn Engl. 50, 1342–1345 (2011).
Seo, Y.H. & Carroll, K.S. Profiling protein thiol oxidation in tumor cells using sulfenic acid-specific antibodies. Proc. Natl. Acad. Sci. USA 106, 16163–16168 (2009).
Acknowledgements
We thank the Packard Foundation, Amgen, Astra Zeneca and Novartis and the US National Institutes of Health (NIH; GM 79465) for providing funding for this work. C.J.C. is an Investigator with the Howard Hughes Medical Institute. B.C.D. was partially supported by a Chemical Biology Training Grant from the NIH (T32 GM066698). Due to space limitations, we apologize if we inadvertently omitted citations of major contributions to this area.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Dickinson, B., Chang, C. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol 7, 504–511 (2011). https://doi.org/10.1038/nchembio.607
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.607
This article is cited by
-
Cadmium toxicity and autophagy: a review
BioMetals (2024)
-
Orally administration of cerium oxide nanozyme for computed tomography imaging and anti-inflammatory/anti-fibrotic therapy of inflammatory bowel disease
Journal of Nanobiotechnology (2023)
-
Self-assembled, hemin-functionalized peptide nanotubes: an innovative strategy for detecting glutathione and glucose molecules with peroxidase-like activity
Nano Convergence (2023)
-
Artificial-enzymes-armed Bifidobacterium longum probiotics for alleviating intestinal inflammation and microbiota dysbiosis
Nature Nanotechnology (2023)
-
NADPH oxidase contributes to the production of reactive oxygen species in Chlorella pyrenoidosa
Biotechnology Letters (2023)