Abstract
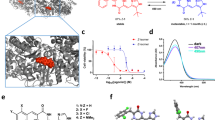
Advances in high-throughput screening now enable the rapid discovery of bioactive small molecules, but these primary hits almost always exhibit modest potency. We report a strategy for the transformation of these hits into much more potent inhibitors without compound optimization. Appending a derivative of Ru(II)(tris-bipyridyl)2+, an efficient photosensitizer of singlet oxygen production, to synthetic protein-binding compounds results in highly potent and specific target protein inactivation upon irradiation with visible light.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Diller, D.J. Curr. Opin. Drug Discov. Devel. 11, 346–355 (2008).
Colas, P. Curr. Drug Discov. Technol. 5, 190–199 (2008).
Davies, M.J. Biochem. Biophys. Res. Commun. 305, 761–770 (2003).
Liao, J.C., Roider, J. & Jay, D.G. Proc. Natl. Acad. Sci. USA 91, 2659–2663 (1994).
Beck, S. et al. Proteomics 2, 247–255 (2002).
Jacobson, K., Rajfur, Z., Vitriol, E. & Hahn, K. Trends Cell Biol. 18, 443–450 (2008).
Winterle, J.S., Kliger, D.S. & Hammond, G.S. J. Am. Chem. Soc. 98, 3719–3721 (1976).
Zhang, X. & Rodgers, M.A.J. J. Phys. Chem. 99, 12797–12803 (1995).
Fuller, Z.J. et al. Anal. Chem. 75, 2670–2677 (2003).
Lee, J., Yu, P., Xiao, X. & Kodadek, T. Mol. Biosyst. 4, 59–65 (2008).
Udugamasooriya, D.G., Dineen, S.P., Brekken, R.A. & Kodadek, T. J. Am. Chem. Soc. 130, 5744–5752 (2008).
Udugamasooriya, D.G., Dunham, G., Ritchie, C., Brekken, R.A. & Kodadek, T. Bioorg. Med. Chem. Lett. 18, 5892–5894 (2008).
Ito, Y., Iwamoto, Y., Tanaka, K., Okuyama, K. & Sugioka, Y. Int. J. Cancer 67, 148–152 (1996).
Wu, W. et al. Mol. Cancer Ther. 6, 471–483 (2007).
Baumeister, W., Walz, J., Zuhl, F. & Seemuller, E. Cell 92, 367–380 (1998).
Smith, D.M. et al. Mol. Cell 20, 687–698 (2005).
Rabl, J. et al. Mol. Cell 30, 360–368 (2008).
Lim, H.S., Archer, C.T. & Kodadek, T. J. Am. Chem. Soc. 129, 7750–7751 (2007).
Lim, H.S., Cai, D., Archer, C.T. & Kodadek, T. J. Am. Chem. Soc. 129, 12936–12937 (2007).
Lim, H.S., Archer, C.T., Kim, Y.C., Hutchens, T. & Kodadek, T. Chem. Commun. (Camb.) 1064–1066 (2008).
Brooks, P. et al. Biochem. J. 346, 155–161 (2000).
Peters, J.M., Franke, W.W. & Kleinschmidt, J.A. J. Biol. Chem. 269, 7709–7718 (1994).
Yogo, T. et al. Chem. Biol. 11, 1053–1058 (2004).
Marks, K.M., Braun, P.D. & Nolan, G.P. Proc. Natl. Acad. Sci. USA 101, 9982–9987 (2004).
Yu, P., Liu, B. & Kodadek, T. Nat. Biotechnol. 23, 746–751 (2005).
Acknowledgements
This work was supported by a contract from the US National Heart, Lung, and Blood Institute (NO1-HV28185) for the University of Texas Southwestern Center for Proteomics Research. We thank M. Rosen (University of Texas Southwestern) and B. Cravatt (The Scripps Research Institute) for a critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
J.L. designed and performed experiments, analyzed data and wrote the manuscript. D.G.U. and H.-S.L. performed experiments. T.K. designed experiments, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figures 1–15 (PDF 827 kb)
Rights and permissions
About this article
Cite this article
Lee, J., Udugamasooriya, D., Lim, HS. et al. Potent and selective photo-inactivation of proteins with peptoid-ruthenium conjugates. Nat Chem Biol 6, 258–260 (2010). https://doi.org/10.1038/nchembio.333
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.333
This article is cited by
-
Ru(II) photocages enable precise control over enzyme activity with red light
Nature Communications (2022)
-
Interrogating biological systems using visible-light-powered catalysis
Nature Reviews Chemistry (2021)
-
Photocatalytic proximity labelling of MCL-1 by a BH3 ligand
Communications Chemistry (2019)
-
Regulation of photosensitisation processes by an RNA aptamer
Scientific Reports (2017)
-
Fragmentation Patterns and Mechanisms of Singly and Doubly Protonated Peptoids Studied by Collision Induced Dissociation
Journal of the American Society for Mass Spectrometry (2016)