Abstract
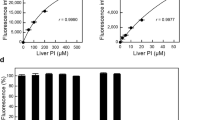
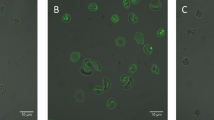
Alkyne-modified phospholipids can be unambiguously identified and differentiated from native species in complex mixtures by formation of dicobalthexacarbonyl complexes. This reaction is specific for alkynes and is unaffected by other glycerophospholipid-related moieties. Enrichment of cells with alkyne-derivatized fatty acids or glycerophospholipids followed by solid-phase sequestration and release is a promising new method for unequivocally monitoring individual glycerophospholipids following incorporation into cells. This technique also facilitates lipidomic analysis of substrates and products.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Walker, S.J. & Brown, H.A. Methods Mol. Biol. 237, 89–97 (2004).
Brown, H.A., Gutowski, S., Moomaw, C.R., Slaughter, C. & Sternweis, P.C. Cell 75, 1137–1144 (1993).
Raetz, C.R. J. Biol. Chem. 251, 3242–3249 (1976).
Ganong, B.R., Leonard, J.M. & Raetz, C.R. J. Biol. Chem. 255, 1923–1929 (1980).
Serunian, L.A., Auger, K.R., Roberts, T.M. & Cantley, L.C. J. Virol. 64, 4718–4725 (1990).
Brown, H.A. & Murphy, R.C. Nat. Chem. Biol. 5, 602–606 (2009).
Zemski Berry, K.A. & Murphy, R.C. J. Lipid Res. 46, 1038–1046 (2005).
Ekroos, K., Chernushevich, I.V., Simons, K. & Shevchenko, A. Anal. Chem. 74, 941–949 (2002).
Nicholas, K.M. & Pettit, R. Tetrahedr. Lett. 12, 3475–3478 (1971).
Seyferth, D., Nestle, M.O. & Wehman, A.T. J. Am. Chem. Soc. 97, 7417–7426 (1975).
Milgrom, L.R., Rees, R.D. & Yahioglu, G. Tetrahedr. Lett. 38, 4905–4908 (1997).
Schreiber, S.L., Sammakia, T. & Crowe, W.E. J. Am. Chem. Soc. 108, 3128–3130 (1986).
Khand, I.U., Knox, G.R., Pauson, P.L. & Watts, W.E. J. Chem. Soc. D: Chem. Commun. 1, 36a (1971).
Khand, I.U., Graham, R.K., Pauson, P.L. & Watts, W.E. J. Chem. Soc. Perkin Trans. 1 1, 975–977 (1973).
Khand, I.U., Knox, G.R., Pauson, P.L., Watts, W.E. & Foreman, M.I. J. Chem. Soc. Perkin Trans. 1 1, 977–981 (1973).
Pauson, P.L. Tetrahedron 41, 5855–5860 (1985).
Ivanova, P.T., Milne, S.B., Forrester, J.S. & Brown, H.A. Mol. Interv. 4, 84–94 (2004).
Milne, S., Ivanova, P., Forrester, J. & Brown, H.A. Methods 39, 92–103 (2006).
Ivanova, P.T., Milne, S.B., Byrne, M.O., Xiang, Y. & Brown, H.A. Methods Enzymol. 432, 21–57 (2007).
Rouzer, C.A. et al. Biochemistry 45, 14795–14808 (2006).
Brown, H.A., Henage, L.G., Preininger, A.M., Xiang, Y. & Exton, J.H. Methods Enzymol. 434, 49–87 (2007).
Comely, A.C., Gibson, S.E. & Hales, N.J. Chem. Commun. (Camb) 2075–2076 (1999).
Comely, A.C., Gibson, S.E., Hales, N.J., Johnstone, C. & Stevenazzi, A. Org. Biomol. Chem. 1, 1959–1968 (2003).
Acknowledgements
The authors thank P. Ivanova and D. Myers for technical assistance and many helpful conversations. This work was partially supported by the US National Institutes of Health (P01-ES013125 to H.A.B., L.J.M. and N.A.P.), a Vanderbilt Institute of Chemical Biology Pilot Program grant (H.A.B. and N.A.P.) and National Institutes of Health Grant Lipid Maps U54 GM69338 (H.A.B.).
Author information
Authors and Affiliations
Contributions
S.B.M. analyzed cell extract samples by mass spectrometry. K.A.T. and R.S. performed chemical synthesis, purification and analysis. S.B.M. and C.M.L. developed the alkyne lipid cobalt derivatization protocol. K.A.T. and S.B.M. developed the capture and release methodology. M.D.A. and C.A.R. contributed to the lipid enrichment procedures. L.J.M., N.A.P. and H.A.B. were responsible for overall project direction and experimental design. All authors discussed the results and modified the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Methods (PDF 801 kb)
Rights and permissions
About this article
Cite this article
Milne, S., Tallman, K., Serwa, R. et al. Capture and release of alkyne-derivatized glycerophospholipids using cobalt chemistry. Nat Chem Biol 6, 205–207 (2010). https://doi.org/10.1038/nchembio.311
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.311
This article is cited by
-
Triglyceride cycling enables modification of stored fatty acids
Nature Metabolism (2023)
-
Synthesis and biological activity of ganglioside GM3 analogues with a (S)-CHF-Sialoside linkage and an alkyne tag
Glycoconjugate Journal (2023)
-
Multiplexed and single cell tracing of lipid metabolism
Nature Methods (2019)
-
Astrocytes and oligodendrocytes in grey and white matter regions of the brain metabolize fatty acids
Scientific Reports (2017)
-
Chemical reporters for biological discovery
Nature Chemical Biology (2013)