Abstract
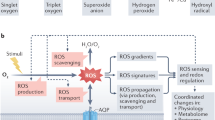
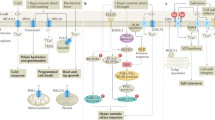
The sessile nature of plants has resulted in the evolution of an extraordinarily diverse suite of protective mechanisms against biotic and abiotic stresses. Though volatile isoprenoids are known to be involved in many types of biotic interactions, they also play important but relatively unappreciated roles in abiotic stress responses. We review those roles, discuss the proposed mechanistic explanations and examine the evolutionary significance of volatile isoprenoid emission. We note that abiotic stress responses generically involve production of reactive oxygen species in plant cells, and volatile isoprenoids mitigate the effects of oxidative stress by mediating the oxidative status of the plant. On the basis of these observations, we propose a 'single biochemical mechanism for multiple physiological stressors' model, whereby the protective effect against abiotic stress is exerted through direct or indirect improvement in resistance to damage by reactive oxygen species.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Intergovernmental Panel on Climate Change. Climate Change 2001: The Scientific Basis (Cambridge University Press, Cambridge, UK, 2001).
Apel, K. & Hirt, H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399 (2004).
Dietz, K.J. Redox control, redox signaling, and redox homeostasis in plant cells. Int. Rev. Cytol. 228, 141–193 (2003).
Kwak, J.M., Nguyen, V. & Schroeder, J.I. The role of reactive oxygen species in hormonal responses. Plant Physiol. 141, 323–329 (2006).
Rohmer, M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 16, 565–574 (1999).
Lange, B.M., Rujan, T., Martin, W. & Croteau, R. Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proc. Natl. Acad. Sci. USA 97, 13172–13177 (2000).
Lichtenthaler, H.K. Non-mevalonate isoprenoid biosynthesis: enzymes, genes and inhibitors. Biochem. Soc. Trans. 28, 785–789 (2000).
Dudareva, N. et al. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc. Natl. Acad. Sci. USA 102, 933–938 (2005).
Laule, O. et al. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 100, 6866–6871 (2003).
Bick, J.A. & Lange, B.M. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch. Biochem. Biophys. 415, 146–154 (2003).
Wu, S.Q. et al. Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nat. Biotechnol. 24, 1441–1447 (2006).
Dudareva, N., Negre, F., Nagegowda, D.A. & Orlova, I. Plant volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 25, 417–440 (2006).
Sharkey, T.D. & Yeh, S. Isoprene emission from plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 407–436 (2001).
Brilli, F. et al. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol. 175, 244–254 (2007).
Loreto, F. & Sharkey, T.D. A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta 182, 523–531 (1990).
Monson, R.K. & Fall, R. Isoprene emission from aspen leaves: influence of environment and relation to photosynthesis and photorespiration. Plant Physiol. 90, 267–274 (1989).
Gershenzon, J. Plant volatiles carry both public and private messages. Proc. Natl. Acad. Sci. USA 104, 5257–5258 (2007).
Baldwin, I.T., Halitschke, R., Paschold, A., von Dahl, C.C. & Preston, C.A. Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science 311, 812–815 (2006).
Loreto, F., Forster, A., Durr, M., Csiky, O. & Seufert, G. On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus ilex L. fumigated with selected monoterpenes. Plant Cell Environ. 21, 101–107 (1998).
Loreto, F., Barta, C., Brilli, F. & Nogues, I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 29, 1820–1828 (2006).
Sharkey, T.D. & Loreto, F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 95, 328–333 (1993).
Tingey, D.T., Evans, R. & Gumpertz, M. Effects of environmental conditions on isoprene emission from live oak. Planta 152, 565–570 (1981).
Filella, I., Peñuelas, J. & Llusia, J. Dynamics of the enhanced emissions of monoterpenes and methyl salicylate, and decreased uptake of formaldehyde, by Quercus ilex leaves after application of jasmonic acid. New Phytol. 169, 135–144 (2006).
Martin, D.M., Gershenzon, J. & Bohlmann, J. Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of norway spruce. Plant Physiol. 132, 1586–1599 (2003).
Schnitzler, J.P. et al. Contribution of different carbon sources to isoprene biosynthesis in poplar leaves. Plant Physiol. 135, 152–160 (2004).
Funk, J.L., Mak, J.E. & Lerdau, M.T. Stress-induced changes in carbon sources for isoprene production in Populus deltoides. Plant Cell Environ. 27, 747–755 (2004).
Rosenstiel, T.N., Ebbets, A.L., Khatri, W.C., Fall, R. & Monson, R.K. Induction of poplar leaf nitrate reductase: a test of extrachloroplastic control of isoprene emission rate. Plant Biol. (Stuttg) 6, 12–21 (2004).
Sanadze, G.A. Biogenic isoprene (a review). Russ. J. Plant Physiol. 51, 729–741 (2004).
Magel, E. et al. Photosynthesis and substrate supply for isoprene biosynthesis in poplar leaves. Atmos. Environ. 40 (suppl. 1): 138–151 (2006).
Sharkey, T.D., Wiberley, A.E. & Donohue, A.R. Isoprene emission from plants: why and how. Ann. Bot. (Lond.) 101, 5–18 (2008).
Laothawornkitkul, J. et al. Isoprene emissions influence herbivore feeding decisions. Plant Cell Environ. 31, 1410–1415 (2008).
Loivamäki, M., Mumm, R., Dicke, M. & Schnitzler, J.P. Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proc. Natl. Acad. Sci. USA 105, 17430–17435 (2008).
Kuzuyama, T., Shimizu, T., Takahashi, S. & Seto, H. Fosmidomycin, a specific inhibitor of 1-deoxy-D-xylulose 5-phosphate reductoisomerase in the nonmevalonate pathway for terpenoid biosynthesis. Tetrahedr. Lett. 39, 7913–7916 (1998).
Sharkey, T.D. & Singsaas, E.L. Why plants emit isoprene. Nature 374, 769 (1995).
Velikova, V., Loreto, F., Tsonev, T., Brilli, F. & Edreva, A. Isoprene prevents the negative consequences of high temperature stress in Platanus orientalis leaves. Funct. Plant Biol. 33, 931–940 (2006).
Velikova, V., Pinelli, P. & Loreto, F. Consequences of inhibition of isoprene synthesis in Phragmites australis leaves exposed to elevated temperatures. Agric. Ecosyst. Environ. 106, 209–217 (2005).
Velikova, V. & Loreto, F. On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ. 28, 318–327 (2005).
Barta, C. & Loreto, F. The relationship between the methyl-erythritol phosphate pathway leading to emission of volatile isoprenoids and abscisic acid content in leaves. Plant Physiol. 141, 1676–1683 (2006).
Loivamäki, M. et al. Arabidopsis, a model to study biological functions of isoprene emission? Plant Physiol. 144, 1066–1078 (2007).
Sasaki, K. et al. Plants utilize isoprene emission as a thermotolerance mechanism. Plant Cell Physiol. 48, 1254–1262 (2007).
Behnke, K. et al. Transgenic, non-isoprene emitting poplars don't like it hot. Plant J. 51, 485–499 (2007).
Molinier, J., Ries, G., Zipfel, C. & Hohn, B. Transgeneration memory of stress in plants. Nature 442, 1046–1049 (2006).
Vickers, C.E. et al. Isoprene synthesis protects transgenic plants from oxidative stress. Plant Cell Environ. published online, doi:10.1111/j.1365–3040.2009.01946.x (22 January 2009).
Delfine, S., Csiky, O., Seufert, G. & Loreto, F. Fumigation with exogenous monoterpenes of a non-isoprenoid-emitting oak (Quercus suber): monoterpene acquisition, translocation, and effect on the photosynthetic properties at high temperatures. New Phytol. 146, 27–36 (2000).
Loreto, F. et al. Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol. 126, 993–1000 (2001).
Loreto, F. & Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 127, 1781–1787 (2001).
Ledford, H.K. & Niyogi, K.K. Singlet oxygen and photo-oxidative stress management in plants and algae. Plant Cell Environ. 28, 1037–1045 (2005).
Affek, H.P. & Yakir, D. Protection by isoprene against singlet oxygen in leaves. Plant Physiol. 129, 269–277 (2002).
Velikova, V., Edreva, A. & Loreto, F. Endogenous isoprene protects Phragmites australis leaves against singlet oxygen. Physiol. Plant. 122, 219–225 (2004).
Loreto, F. & Fares, S. Is ozone flux Inside leaves only a damage indicator? Clues from volatile isoprenoid studies. Plant Physiol. 143, 1096–1100 (2007).
Calogirou, A., Larsen, B.R. & Kotzias, D. Gas-phase terpene oxidation products: a review. Atmos. Environ. 33, 1423–1439 (1999).
Duhl, T.R., Helmig, D. & Guenther, A. Sesquiterpene emissions from vegetation: a review. Biogeosciences Discuss. 4, 3987–4023 (2007).
Helmig, D., Bocquet, F., Pollmann, J. & Revermann, T. Analytical techniques for sesquiterpene emission rate studies in vegetation enclosure experiments. Atmos. Environ. 38, 557–572 (2004).
Siwko, M.E. et al. Does isoprene protect plant membranes from thermal shock? A molecular dynamics study. Biochim. Biophys. Acta 1768, 198–206 (2007).
Milne, P.J., Riemer, D.D., Zika, R.G. & Brand, L.E. Measurement of vertical distribution of isoprene in surface seawater, its chemical fate, and its emission from several phytoplankton monocultures. Mar. Chem. 48, 237–244 (1995).
Logan, B.A. & Monson, R.K. Thermotolerance of leaf discs from four isoprene-emitting species is not enhanced by exposure to exogenous isoprene. Plant Physiol. 120, 821–826 (1999).
Logan, B.A., Anchordoquy, T.J., Monson, R.K. & Pan, R.S. The effect of isoprene on the properties of spinach thylakoids and phosphatidylcholine liposomes. Plant Biol. (Stuttg) 1, 602–606 (1999).
Velikova, V. et al. Isoprene decreases the concentration of nitric oxide in leaves exposed to elevated ozone. New Phytol. 166, 419–425 (2005).
Thompson, A.M. The oxidizing capacity of the earth's atmosphere - probable past and future changes. Science 256, 1157–1165 (1992).
Monson, R.K. & Holland, E.A. Biospheric trace gas fluxes and their control over tropospheric chemistry. Annu. Rev. Ecol. Syst. 32, 547–576 (2001).
Pierce, T. et al. Influence of increased isoprene emissions on regional ozone modeling. J. Geophys. Res. Atmos. 103, 25611–25629 (1998).
Mullineaux, P.M., Karpinski, S. & Baker, N.R. Spatial dependence for hydrogen peroxide-directed signaling in light-stressed plants. Plant Physiol. 141, 346–350 (2006).
Mullineaux, P. et al. Are diverse signalling pathways integrated in the regulation of Arabidopsis antioxidant defence gene expression in response to excess excitation energy? Phil. Trans. R. Soc. Lond. B 355, 1531–1540 (2000).
Munné-Bosch, S. & Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 21, 31–57 (2002).
Sauer, F., Schäfer, C., Neeb, P., Horie, O. & Moorgat, G.K. Formation of hydrogen peroxide in the ozonolysis of isoprene and and simple alkenes under humid conditions. Atmos. Environ. 33, 229–241 (1999).
Fares, S., Loreto, F., Kleist, E. & Wildt, J. Stomatal uptake and stomatal deposition of ozone in isoprene and monoterpene emitting plants. Plant Biol. (Stuttg) 10, 44–54 (2008).
Alméras, E. et al. Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 34, 205–216 (2003).
Santos, L.S., Dalmazio, I., Eberlin, M.N., Claeys, M. & Augusti, R. Mimicking the atmospheric OH-radical-mediated photooxidation of isoprene: formation of cloud-condensation nuclei polyols monitored by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 20, 2104–2108 (2006).
Zeidler, J.G., Lichtenthaler, H.K., May, H.U. & Lichtenthaler, F.W. Is isoprene emitted by plants synthesized via the novel isopentenyl pyrophosphate pathway? Z. Naturforsch. 52c, 15–23 (1997).
Foyer, C., Trebst, A. & Noctor, G. Signaling and integration of defense functions of tocopherol, ascorbate and glutathione. in Photoprotection, Photoinhibition, Gene Regulation, and Environment (eds. Demmig-Adams, B., Adams, W.W. III & Mattoo, A.K.) 241–268 (Springer, Dordrecht, The Netherlands, 2006).
Triantaphylides, C. et al. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 148, 960–968 (2008).
Pasqualini, S. et al. Ozone-induced cell death in tobacco cultivar Bel W3 plants. The role of programmed cell death in lesion formation. Plant Physiol. 133, 1122–1134 (2003).
Gould, K.S., Lamotte, O., Klinguer, A., Pugin, A. & Wendehenne, D. Nitric oxide production in tobacco leaf cells: a generalized stress response? Plant Cell Environ. 26, 1851–1862 (2003).
Wilson, I.D., Neill, S.J. & Hancock, J.T. Nitric oxide synthesis and signalling in plants. Plant Cell Environ. 31, 622–631 (2008).
Beligni, M.V. & Lamattina, L. Nitric oxide interferes with plant photo-oxidative stress by detoxifying reactive oxygen species. Plant Cell Environ. 25, 737–748 (2002).
Velikova, V., Fares, S. & Loreto, F. Isoprene and nitric oxide reduce damages in leaves exposed to oxidative stress. Plant Cell Environ. 31, 1882–1894 (2008).
Harley, P.C., Monson, R.K. & Lerdau, M.T. Ecological and evolutionary aspects of isoprene emission from plants. Oecologia 118, 109–123 (1999).
Sharkey, T.D. et al. Evolution of the isoprene biosynthetic pathway in kudzu. Plant Physiol. 137, 700–712 (2005).
Harley, P., Guenther, A. & Zimmerman, P. Environmental controls over isoprene emission in deciduous oak canopies. Tree Physiol. 17, 705–714 (1997).
Loreto, F. et al. Different sources of reduced carbon contribute to form three classes of terpenoid emitted by Quercus ilex L. leaves. Proc. Natl. Acad. Sci. USA 93, 9966–9969 (1996).
Niinemets, U., Loreto, F. & Reichstein, M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 9, 180–186 (2004).
Loreto, F. Distribution of isoprenoid emitters in the Quercus genus around the world: chemo-taxonomical implications and evolutionary considerations based on the ecological function of the trait. Perspect. Plant Ecol. Evol. Syst. 5, 185–192 (2002).
Lerdau, M. A positive feedback with negative consequences. Science 316, 212–213 (2007).
Rosenstiel, T.N., Potosnak, M.J., Griffin, K.L., Fall, R. & Monson, R.K. Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature 421, 256–259 (2003).
Loreto, F. et al. The relationship between isoprene emission rate and dark respiration rate in white poplar (Populus alba L.) leaves. Plant Cell Environ. 30, 662–669 (2007).
Scheel, D. & Wasternac, C. Plant Signal Transduction (Oxford University Press, Oxford, 2002).
Schilmiller, A.L. & Howe, G.A. Systemic signaling in the wound response. Curr. Opin. Plant Biol. 8, 369–377 (2005).
Jenks, M.A. & Hasegawa, P.M. Plant Abiotic Stress (Blackwell, Oxford, 2005).
Hirt, H. & Shinozaki, K. Plant Responses to Abiotic Stress (Springer, Berlin, 2004).
Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53, 247–273 (2002).
Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 56, 337–346 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vickers, C., Gershenzon, J., Lerdau, M. et al. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 5, 283–291 (2009). https://doi.org/10.1038/nchembio.158
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.158
This article is cited by
-
Exploring the versatility of sesquiterpene biosynthesis in guava plants: a comparative genome-wide analysis of two cultivars
Scientific Reports (2024)
-
Atmospheric Degradation of Ecologically Important Biogenic Volatiles: Investigating the Ozonolysis of (E)-β-Ocimene, Isomers of α and β-Farnesene, α-Terpinene and 6-Methyl-5-Hepten-2-One, and Their Gas-Phase Products
Journal of Chemical Ecology (2024)
-
Genome-wide identification, expression profile and evolutionary relationships of TPS genes in the neotropical fruit tree species Psidium cattleyanum
Scientific Reports (2023)
-
Overexpression of a Flavonol Synthase Gene from Apocynum venetum Improves the Salinity Stress Tolerance of Transgenic Arabidopsis thaliana
Journal of Soil Science and Plant Nutrition (2023)
-
Quality trait improvement in horticultural crops: OMICS and modern biotechnological approaches
Molecular Biology Reports (2023)