Abstract
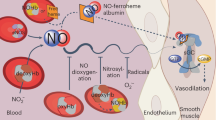
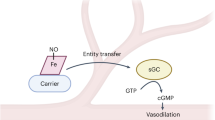
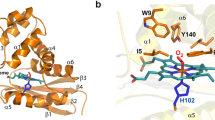
Nitrite reacts with deoxyhemoglobin to form nitric oxide (NO) and methemoglobin. Though this reaction is experimentally associated with NO generation and vasodilation, kinetic analysis suggests that NO should not be able to escape inactivation in the erythrocyte. We have discovered that products of the nitrite-hemoglobin reaction generate dinitrogen trioxide (N2O3) via a novel reaction of NO and nitrite-bound methemoglobin. The oxygen-bound form of nitrite-methemoglobin shows a degree of ferrous nitrogen dioxide (Fe(II)-NO2˙) character, so it may rapidly react with NO to form N2O3. N2O3 partitions in lipid, homolyzes to NO and readily nitrosates thiols, all of which are common pathways for NO escape from the erythrocyte. These results reveal a fundamental heme globin– and nitrite-catalyzed chemical reaction pathway to N2O3, NO and S-nitrosothiol that could form the basis of in vivo nitrite-dependent signaling. Because the reaction redox-cycles (that is, regenerates ferrous heme) and the nitrite-methemoglobin intermediate is not observable by electron paramagnetic resonance spectroscopy, this reaction has been 'invisible' to experimentalists over the last 100 years.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gladwin, M.T. et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc. Natl. Acad. Sci. USA 97, 11482–11487 (2000).
Cosby, K. et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 9, 1498–1505 (2003).
Gladwin, M.T. et al. The emerging biology of the nitrite anion. Nat. Chem. Biol. 1, 308–314 (2005).
Bryan, N.S. et al. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat. Chem. Biol. 1, 290–297 (2005).
Modin, A. et al. Nitrite-derived nitric oxide: a possible mediator of 'acidic-metabolic' vasodilation. Acta Physiol. Scand. 171, 9–16 (2001).
Huang, K.T. et al. The reaction between nitrite and deoxyhemoglobin: reassessment of reaction kinetics and stoichiometry. J. Biol. Chem. 280, 31126–31131 (2005).
Huang, Z. et al. Enzymatic function of hemoglobin as a nitrite reductase that produces nitric oxide under allosteric control. J. Clin. Invest. 115, 2099–2107 (2005).
Dejam, A. et al. Nitrite infusion in humans and nonhuman primates. Endocrine effects, pharmacokinetics, and tolerance formation. Circulation 116, 1821–1831 (2007).
Enemark, J.H. & Feltham, R.D. Principles of structure, bonding, and reactivity for metal nitrosyl complexes. Coord. Chem. Rev. 13, 339–406 (1974).
Crawford, J.H. et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood 107, 566–574 (2006).
Nagababu, E., Ramasamy, S., Abernethy, D.R. & Rifkind, J.M. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J. Biol. Chem. 278, 46349–46356 (2003).
Jeffers, A. et al. Hemoglobin mediated nitrite activation of soluble guanylyl cyclase. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 142, 130–135 (2005).
Shiva, S. et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 100, 654–661 (2007).
Rassaf, T. et al. Nitrite reductase function of deoxymyoglobin - oxygen sensor and regulator of cardiac energetics and function. Circ. Res. 100, 1749–1754 (2007).
Robinson, J.M. & Lancaster, J.R. Hemoglobin-mediated, hypoxia-induced vasodilation via nitric oxide - mechanism(s) and physiologic versus pathophysiologic relevance. Am. J. Respir. Cell Mol. Biol. 32, 257–261 (2005).
Williams, D.L.H. Nitrosation Reactions and the Chemsitry of Nitric Oxide 5–8 (Elsevier, Amsterdam, 2004).
Wink, D.A., Darbyshire, J.F., Nims, R.W., Saavedra, J.E. & Ford, P.C. Reactions of the bioregulatory agent nitric-oxide in oxygenated aqueous-media - determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem. Res. Toxicol. 6, 23–27 (1993).
Dabora, R., Molina, M., Ng, V., Wishnok, J.S. & Tannenbaum, S.R. Nitrosation by alkyl nitrites - catalysis by inorganic salts. IARC Sci. Publ. 57, 311–316 (1984).
Stepuro, I.I., Chaikovskaya, N.A., Solodunov, A.A. & Artsukevich, A.N. Generation of NO during oxidation of hemoglobin ferroforms by nitrite. Biochem. Moscow 62, 960–966 (1997).
Luchsinger, B.P. et al. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc. Natl. Acad. Sci. USA 100, 461–466 (2003).
Fernandez, B.O. & Ford, P.C. Nitrite catalyzes ferriheme protein reductive nitrosylation. J. Am. Chem. Soc. 125, 10510–10511 (2003).
Nagababu, E., Ramasamy, S. & Rifkind, J.M. S-Nitrosohemoglobin: a mechanism for its formation in conjunction with nitrite reduction by deoxyhemoglobin. Nitric Oxide 15, 20–29 (2006).
Angelo, M., Singel, D.J. & Stamler, J.S. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc. Natl. Acad. Sci. USA 103, 8366–8371 (2006).
Day, E.P. et al. Magnetization of the sulfite and nitrite complexes of oxidized sulfite and nitrite reductases - electron-paramagnetic-res silent spin S = 1/2 states. Biochemistry 27, 2126–2132 (1988).
Young, L.J. & Siegel, L.M. On the reaction of ferric heme-proteins with nitrite and sulfite. Biochemistry 27, 2790–2800 (1988).
Rodkey, F.L. Mechanism for conversion of oxyhemoglobin to methemoglobin by nitrite. Clin. Chem. 22, 1986–1990 (1976).
Wanat, A. et al. Nitrite binding to metmyoglobin and methemoglobin in comparison to nitric oxide binding. J. Biol. Inorg. Chem. 7, 165–176 (2002).
Conradie, J. & Ghosh, A. Iron(iii)-nitro porphyrins: theoretical exploration of a unique class of reactive molecules. Inorg. Chem. 45, 4902–4909 (2006).
O'Shea, S.K., Wang, W., Wade, R.S. & Castro, C.E. Selective oxygen transfers with iron(iii) porphyrin nitrite. J. Org. Chem. 61, 6388–6395 (1996).
Castro, C.E. & Oshea, S.K. Activation of nitrite ion by iron(III) porphyrins - stoichiometric oxygen-transfer to carbon, nitrogen, phosphorus, and sulfur. J. Org. Chem. 60, 1922–1923 (1995).
Nasri, H., Ellison, M.K., Shang, M.Y., Schulz, C.E. & Scheidt, W.R. Variable pi-bonding in iron(ii) porphyrinates with nitrite, CO, and tert-butyl isocyanide: characterization of [Fe(TpivPP)(NO2)(CO)](-). Inorg. Chem. 43, 2932–2942 (2004).
Lim, M.D. et al. Reactions of nitrogen oxides with heme models. Characterization of NO and NO2 dissociation from Fe(TPP)(NO2)(NO) by flash photolysis and rapid dilution techniques: Fe(TPP)(NO2) as an unstable intermediate. J. Am. Chem. Soc. 124, 9737–9743 (2002).
Han, T.H., Hyduke, D.R., Vaughn, M.W., Fukuto, J.M. & Liao, J.C. Nitric oxide reaction with red blood cells and hemoglobin under heterogeneous conditions. Proc. Natl. Acad. Sci. USA 99, 7763–7768 (2002).
Cooper, C.E. Nitric oxide and iron proteins. Biochim. Biophys. Acta 1411, 290–309 (1999).
Hoshino, M., Maeda, M., Konishi, R., Seki, H. & Ford, P.C. Studies on the reaction mechanism for reductive nitrosylation of ferrihemoproteins in buffer solutions. J. Am. Chem. Soc. 118, 5702–5707 (1996).
Lauer, T. et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc. Natl. Acad. Sci. USA 98, 12814–12819 (2001).
Singel, D.J. & Stamler, J.S. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu. Rev. Physiol. 67, 99–145 (2005).
Huang, K.T., Azarov, I., Basu, S., Huang, J. & Kim-Shapiro, D.B. Lack of allosterically controlled intramolecular transfer of nitric oxide from the heme to cysteine in the beta subunit of hemoglobin. Blood 107, 2602–2604 (2006).
Doyle, M.P. & Hoekstra, J.W. Oxidation of nitrogen-oxides by bound dioxygen in hemoproteins. J. Inorg. Biochem. 14, 351–358 (1981).
Eich, R.F. et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry 35, 6976–6983 (1996).
Herold, S., Exner, M. & Nauser, T. Kinetic and mechanistic studies of the NO center dot-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry 40, 3385–3395 (2001).
Kim-Shapiro, D.B. Hemoglobin-nitric oxide cooperativity: is NO the third respiratory ligand? Free Radic. Biol. Med. 36, 402–412 (2004).
Copeland, D.M., Soares, A.S., West, A.H. & Richter-Addo, G.B. Crystal structures of the nitrite and nitric oxide complexes of horse heart myoglobin. J. Inorg. Biochem. 100, 1413–1425 (2006).
Espey, M.G., Miranda, K.M., Thomas, D.D. & Wink, D.A. Distinction between nitrosating mechanisms within human cells and aqueous solution. J. Biol. Chem. 276, 30085–30091 (2001).
Challis, B.C. & Kyrtopoulos, S.A. Chemistry of nitroso-compounds. 12. Mechanism of nitrosation and nitration of aqueous piperidine by gaseous dinitrogen tetraoxide and dinitrogen trioxide in aqueous alkaline-solutions - evidence for the existence of molecular isomers of dinitrogen tetraoxide and dinitrogen trioxide. J. Chem. Soc. [Perkin] 2, 1296–1302 (1978).
Gladwin, M.T., Crawford, J.H. & Patel, R.P. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic. Biol. Med. 36, 707–717 (2004).
Shiva, S. et al. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat. Chem. Biol. 2, 486–493 (2006).
Fang, K.Z., Ragsdale, N.V., Carey, R.M., MacDonald, T. & Gaston, B. Reductive assays for S-nitrosothiols: implications for measurements in biological systems. Biochem. Biophys. Res. Commun. 252, 535–540 (1998).
Yang, B.K., Vivas, E.X., Reiter, C.D. & Gladwin, M.T. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic. Res. 37, 1–10 (2003).
Handy, N.C. & Cohen, A.J. Left-right correlation energy. Mol. Phys. 99, 403–412 (2001).
Lee, C.T., Yang, W.T. & Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron-density. Phys. Rev. B Condens. Matter 37, 785–789 (1988).
Acknowledgements
We thank D.L.H. Williams for helpful discussion and for his seminal and pioneering work in nitrosation chemistry. This work was supported by US National Institutes of Health (NIH) grants HL58091 (D.B.K.-S.), GM55792 (N.H.) and HL62198 (S.B.K.). EPR spectrometry was facilitated by a grant from the North Carolina Biotechnology Center (2003-IDG-1013, D.B.K.-S.). D.B.K.-S. gratefully acknowledges further support from NIH grant K02 HL078706. A.G. thanks the Research Council of Norway for supercomputing time, and J.C. thanks the South African National Research Foundation for further support. M.T.G. is supported by the Division of Intramural Research of the US National Heart, Lung, and Blood Institute.
Author information
Authors and Affiliations
Contributions
The bulk of the experiments were performed by S.B. and R.G., who also helped in the design of these experiments and in writing the paper. A.G. and J.C. performed DFT calculations and wrote the text associated with these. D.B.K.-S. and M.T.G. directed the research, designed the experiments and wrote most of the paper. Significant other intellectual contributions (and some writing) were made by R.P., S.B.K and N.H. Other experiments were performed by J.H., A. Jeffers, A. Jiang, X.H., I.A., R.S., Z.H. and A.M.
Corresponding authors
Ethics declarations
Competing interests
R.P., M.T.G. and D.B.K.-S. are listed as coinventors on US National Institutes of Health government patent applications entitled “Use of Nitrite Salts for the Treatment of Cardiovascular Conditions” and “Nitrite-Methemoglobin therapy to detoxify stroma-free hemoglobin based blood substitutes.”
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Methods (PDF 410 kb)
Rights and permissions
About this article
Cite this article
Basu, S., Grubina, R., Huang, J. et al. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol 3, 785–794 (2007). https://doi.org/10.1038/nchembio.2007.46
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2007.46
This article is cited by
-
Thiol-catalyzed formation of NO-ferroheme regulates intravascular NO signaling
Nature Chemical Biology (2023)
-
Can erythrocytes release biologically active NO?
Cell Communication and Signaling (2016)
-
Brief inhalation of nitric oxide increases resuscitation success and improves 7-day-survival after cardiac arrest in rats: a randomized controlled animal study
Critical Care (2015)
-
Convergence of biological nitration and nitrosation via symmetrical nitrous anhydride
Nature Chemical Biology (2015)
-
Quantitative Systems Pharmacology Model of NO Metabolome and Methemoglobin Following Long‐Term Infusion of Sodium Nitrite in Humans
CPT: Pharmacometrics & Systems Pharmacology (2013)